The Project Gutenberg EBook of Facts and Arguments for Darwin, by Fritz Müller
This eBook is for the use of anyone anywhere in the United States and most
other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms of
the Project Gutenberg License included with this eBook or online at
www.gutenberg.org. If you are not located in the United States, you'll have
to check the laws of the country where you are located before using this ebook.
Title: Facts and Arguments for Darwin
Author: Fritz Müller
Release Date: December 19, 2002 [EBook #6475]
[Most recently updated: April 10, 2020]
Language: English
Character set encoding: UTF-8
*** START OF THIS PROJECT GUTENBERG EBOOK FACTS AND ARGUMENTS FOR DARWIN ***
Produced by Sue Asscher
Facts and Arguments for Darwin
by Fritz Müller
WITH ADDITIONS BY THE AUTHOR
Translated from the German
by W. S. DALLAS, F.L.S.
Assistant Secretary to the Geological Society of London
WITH ILLUSTRATIONS
LONDON:
JOHN MURRAY, ALBEMARLE
STREET
1869
Mr. DARWIN'S WORKS
A NATURALIST’S VOYAGE ROUND THE WORLD; being a Journal of Researches
into the Natural History and Geology of Countries visited. Post 8vo. 9
shillings.
THE ORIGIN OF SPECIES, BY MEANS OF NATURAL SELECTION; or, the Preservation
of Favoured Races in the Struggle for Life. Woodcuts. Post 8vo. 15
shillings.
THE VARIOUS CONTRIVANCES BY WHICH BRITISH AND FOREIGN ORCHIDS ARE
FERTILISED BY INSECTS, and on the Good Effects of Intercrossing. Woodcuts,
Post 8vo. 9 shillings.
THE VARIATION OF ANIMALS AND PLANTS UNDER DOMESTICATION. Illustrations. 2
volumes, 8vo. 28 shillings.
Contents
TRANSLATOR’S PREFACE
My principal reason for undertaking the translation of Dr. Fritz Müller’s
admirable work on the Crustacea, entitled ‘Für Darwin,’
was that it was still, although published as long ago as 1864, and highly
esteemed by the author’s scientific countrymen, absolutely unknown
to a great number of English naturalists, including some who have occupied
themselves more or less specially with the subjects of which it treats. It
possesses a value quite independent of its reference to Darwinism, due to
the number of highly interesting and important facts in the natural
history and particularly the developmental history of the Crustacea, which
its distinguished author, himself an unwearied and original investigator
of these matters, has brought together in it. To a considerable section of
English naturalists the tone adopted by the author in speaking of one of
the greatest of their number will be a source of much gratification.
In granting his permission for the translation of his little book, Dr.
Fritz Müller kindly offered to send some emendations and additions to
certain parts of it. His notes included many corrections of printers'
errors, some of which would have proved unintelligible without his aid,
some small additions and notes which have been inserted in their proper
places, and two longer pieces, one forming a footnote near the close of
Chapter 11, the other at the end of Chapter 12, describing the probable
mode of evolution of the Rhizocephala from the Cirripedia.
Of the execution of the translation I will say but little. My chief object
in this, as in other cases, has been to furnish, as nearly as possible, a
literal version of the original, regarding mere elegance of expression as
of secondary importance in a scientific work. As much of Dr. Müller’s
German does not submit itself to such treatment very readily, I must beg
his and the reader’s indulgence for any imperfections arising from
this cause.
W.S.D.
LONDON, 15th February, 1869.
AUTHOR’S PREFACE
It is not the purpose of the following pages to discuss once more the
arguments deduced for and against Darwin’s theory of the origin of
species, or to weigh them one against the other. Their object is simply to
indicate a few facts favourable to this theory, collected upon the same
South American ground, on which, as Darwin tells us, the idea first
occurred to him of devoting his attention to “the origin of species,—that
mystery of mysteries.”
It is only by the accumulation of new and valuable material that the
controversy will gradually be brought into a state fit for final decision,
and this appears to be for the present of more importance than a repeated
analysis of what is already before us. Moreover, it is but fair to leave
it to Darwin himself at first to beat off the attacks of his opponents
from the splendid structure which he has raised with such a master-hand.
F.M.
DESTERRO, 7th September, 1863.
HISTORY OF CRUSTACEA
CHAPTER I.
INTRODUCTORY.
When I had read Charles Darwin's book ‘On the Origin of Species,’
it seemed to me that there was one mode, and that perhaps the most certain, of
testing the correctness of the views developed in it, namely, to attempt to
apply them as specially as possible to some particular group of animals. such
an attempt to establish a genealogical tree, whether for the families of a
class, the genera of a large family, or for the species of an extensive genus,
and to produce pictures as complete and intelligible as possible of the common
ancestors of the various smaller and larger circles, might furnish a result in
three different ways.
1. In the first place, Darwin’s suppositions when thus applied might lead
to irreconcilable and contradictory conclusions, from which the erroneousness
of the suppositions might be inferred. If Darwin’s opinions are false, it
was to be expected that contradictions would accompany their detailed
application at every step, and that these, by their cumulative force, would
entirely destroy the suppositions from which they proceeded, even though the
deductions derived from each particular case might possess little of the
unconditional nature of mathematical proof.
2. Secondly, the attempt might be successful to a greater or less extent. If it
was possible upon the foundation and with the aid of the Darwinian theory, to
show in what sequence the various smaller and larger circles had separated from
the common fundamental form and from each other, in what sequence they had
acquired the peculiarities which now characterise them, and what
transformations they had undergone in the lapse of ages,—if the
establishment of such a genealogical tree, of a primitive history of the group
under consideration, free from internal contradictions, was
possible,—then this conception, the more completely it took up all the
species within itself, and the more deeply it enabled us to descend into the
details of their structure, must in the same proportion bear in itself the
warrant of its truth, and the more convincingly prove that the foundation upon
which it is built is no loose sand, and that it is more than merely “an
intellectual dream.”
3. In the third place, however, it was possible, and this could not but appear,
primâ facie, the most probable case, that the attempt might be
frustrated by the difficulties standing in its way, without settling the
question, either way, in a perfectly satisfactory manner. But if it were only
possible in this way to arrive for oneself at a moderately certain independent
judgment upon a matter affecting the highest questions so deeply, even this
alone could not but be esteemed a great gain.
Having determined to make the attempt, I had in the first place to decide upon
some particular class. The choice was necessarily limited to those the chief
forms of which were easily to be obtained alive in some abundance. The Crabs
and Macrurous Crustacea, the Stomapoda, the Diastylidæ, the Amphipoda and
Isopoda, the Ostracoda and Daphnidæ, the Copepoda and Parasita, the Cirripedes
and Rhizocephala of our coast, representing the class of Crustacea with the
deficiency only of the Phyllopoda and Xiphosura, furnished a long and varied,
and at the same time intimately connected series, such as was at my command in
no other class. But even independently of this circumstance the selection of
the Crustacea could hardly have been doubtful. Nowhere else, as has already
been indicated by various writers, is the temptation stronger to give to the
expressions “relationship, production from a common fundamental
form,” and the like, more than a mere figurative signification, than in
the case of the lower Crustacea. Among the parasitic Crustacea, especially,
everybody has long been accustomed to speak, in a manner scarcely admitting of
a figurative meaning, of their arrest of development by parasitism, as if the
transformation of species were a matter of course. It would certainly never
appear to any one to be a pastime worthy of the Deity, to amuse himself with
the contrivance of these marvellous cripplings, and so they were supposed to
have fallen by their own fault, like Adam, from their previous state of
perfection.
That a great part of the larger and smaller groups into which this class is
divided, might be regarded as satisfactorily established, was a further
advantage not to be undervalued; whilst in two other classes with which I was
familiar, namely, the Annelida and Acalephæ, all the attempted arrangements
could only be considered preliminary revisions. These undisplaceable groups,
like the sharply marked forms of the hard, many-jointed dermal framework, were
not only important as safe starting points and supports, but were also of the
highest value as inflexible barriers in a problem in which, from its very
nature, fancy must freely unfold her wings.
When I thus began to study our Crustacea more closely from this new stand-point
of the Darwinian theory,—when I attempted to bring their arrangements
into the form of a geological tree, and to form some idea of the probable
structure of their ancestors,—I speedily saw (as indeed I expected) that
it would require years of preliminary work before the essential problem could
be seriously handled. The extant systematic works generally laid more weight
upon the characters separating the genera, families and orders, than upon those
which unite the members of each group, and consequently often furnished but
little employable material. But above all things a thorough knowledge of
development was indispensable, and every one knows how imperfect is our present
knowledge of this subject. The existing deficiencies were the more difficult to
supply, because, as Van Beneden remarks with regard to the Decapoda, from the
often incredible difference in the development of the most nearly allied forms,
these must be separately studied—usually family by family, and frequently
genus by genus—nay, sometimes, as in the case of Penëus, even
species by species; and because these investigations, in themselves troublesome
and tedious, often depend for their success upon a lucky chance.
But although the satisfactory completion of the “Genealogical tree of the
Crustacea” appeared to be an undertaking for which the strength and life
of an individual would hardly suffice, even under more favourable circumstances
than could be presented by a distant island, far removed from the great market
of scientific life, far from libraries and museums—nevertheless its
practicability became daily less doubtful in my eyes, and fresh observations
daily made me more favourably inclined towards the Darwinian theory.
In determining to state the arguments which I derived from the consideration of
our Crustacea in favour of Darwin’s views, and which (together with more
general considerations and observations in other departments), essentially
aided in making the correctness of those views seem more and more palpable to
me, I am chiefly influenced by an expression of Darwin’s:
“Whoever,” says he (‘Origin of Species’ page 482),
“is led to believe that species are mutable, will do a good service by
conscientiously expressing his conviction.” To the desire expressed in
these words I respond, for my own part, with the more pleasure, as this
furnishes me with an opportunity of publicly giving expression in words to the
thanks which I feel most deeply to be due from me to Darwin for the
instructions and suggestions for which I am so deeply indebted to his book.
Accordingly I throw this sand-grain with confidence into the scale against
“the load of prejudice by which this subject is overwhelmed,”
without troubling myself as to whether the priests of orthodox science will
reckon me amongst dreamers and children in knowledge of the laws of nature.
CHAPTER II.
THE SPECIES OF MELITA.
A false supposition, when the consequences
proceeding from it are followed further and further, will sooner or
later lead to absurdities and palpable contradictions. During the
period of tormenting doubt—and this was by no means a short
one—when the pointer of the scales oscillated before me in
perfect uncertainty between the pro and the con, and
when any fact leading to a quick decision would have been most
welcome to me, I took no small pains to detect some such
contradictions among the inferences as to the class of Crustacea
furnished by the Darwinian theory. But I found none, either then,
or subsequently. Those which I thought I had found were dispelled
on closer consideration, or actually became converted into supports
for Darwin’s theory.
Nor, so far as I am aware, have any of the necessary
consequences of Darwin’s hypotheses been proved by any one
else, to stand in clear and irreconcilable contradiction. And yet,
as the most profound students of the animal kingdom are amongst
Darwin’s opponents, it would seem that it ought to have been
an easy matter for them to crush him long since beneath a mass of
absurd and contradictory inferences, if any such were to be drawn
from his theory. To this want of demonstrated contradictions I
think we may ascribe just the same importance in Darwin’s
favour, that his opponents have attributed to the absence of
demonstrated intermediate forms between the species of the various
strata of the earth. Independently of the reasons which Darwin
gives for the preservation of such intermediate forms being only
exceptional, this last mentioned circumstance will not be regarded
as of very great significance by any one who has traced the
development of an animal upon larvae fished from the sea, and had
to seek in vain for months, and even years, for those transitional
forms, which he nevertheless knew to be swarming around him in
thousands.
A few examples may show how contradictions might come forth as
necessary results of the Darwinian hypotheses.
It seems to be a necessity for all crabs which remain for a long
time out of the water (but why is of no consequence to us here),
that air shall penetrate from behind into the branchial cavity. Now
these crabs, which have become more or less estranged from the
water, belong to the most different families—the
Raninidæ (Ranina), Eriphinæ (Eriphia
gonagra), Grapsoidæ (Aratus, Sesarma, etc.),
Ocypodidæ (Gelasimus, Ocypoda), etc., and the
separation of these families must doubtless be referred to a much
earlier period than the habit of leaving the water displayed by
some of their members. The arrangements connected with aerial
respiration, therefore, could not be inherited from a common
ancestor, and could scarcely be accordant in their construction. If
there were any such accordance not referable to accidental
resemblance among them, it would have to be laid in the scale as
evidence against the correctness of Darwin’s views. I shall
show hereafter how in this case the result, far from presenting
such contradictions, was rather in the most complete harmony with
what might be predicted from Darwin’s theory.
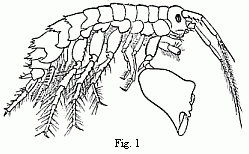
Fig. 1. Melita exilii n. sp., male, enlarged. The
large branchial lamellæ are seen projecting between the legs.
A second example.—We are already acquainted with four
species of Melita (M. valida, setipes, anisochir, and
Fresnelii), and I can add a fifth (Fig. 1), in which the
second pair of feet bears upon one side a small hand of the usual
structure, and on the other an enormous clasp-forceps. This want of
symmetry is something so unusual among the Amphipoda, and the
structure of the clasp-forceps differs so much from what is seen
elsewhere in this order, and agrees so closely in the five species,
that one must unhesitatingly regard them as having sprung from
common ancestors belonging to them alone among known species. But
one of these species, M. Fresnelii, discovered by Savigny,
in Egypt, is said to want the secondary flagellum of the anterior
antennae, which occurs in the others. From the trustworthiness of
all Savigny’s works there can scarcely be a doubt as to the
correctness of this statement. Now, if the presence or absence of
the secondary flagellum possessed the significance of a distinctive
generic character, which is usually ascribed to it, or if there
were other important differences between Melita Fresnelii
and the other species above-mentioned, which would make it seem
natural to separate M. Fresnelii as a distinct genus, and to
leave the others united with the rest of the species of
Melita—that is to say, in the sense of the Darwinian
theory, if we assume that all the other Melitæ
possessed common ancestors, which were not at the same time the
ancestors of M. Fresnelii—this would stand in
contradiction to the conclusion, derived from the structure of the
clasp-forceps, that M. Fresnelii and the four other species
above-mentioned possessed common ancestors, which were not also the
ancestors of the remaining species of Melita. It would
follow—
| From the structure of the clasp-forceps: |
From the presence or absence of the
secondary flagellum. |
 |
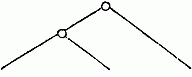 |
| M. palmata, etc., M. exilii, etc., M. Fresnelli. |
M. palmata, etc., M. exilii, etc., M. Fresnelii. |
As, in the first case, among the Crabs, a typical agreement of arrangements
produced independently of each other would have been a very suspicious
circumstance for Darwin’s theory, so also, in the second, would any
difference more profound than that of very nearly allied species. Now it seems
to me that the secondary flagellum can by no means furnish a reason for
doubting the close relationship of M. Fresnelii to M. exilii,
etc., which is indicated by the peculiar structure of the unpaired
clasp-forceps. In the first place we must consider the possibility that the
secondary flagellum, which is not always easy to detect, may only have been
overlooked by Savigny, as indeed Spence Bate supposes to have been the case. If
it is really deficient it must be remarked that I have found it in species of
the genera Leucothoë, Cyrtophium and Amphilochus, in which
genera it was missed by Savigny, Dana and Spence Bate—that a species
proved by the form of the Epimera (coxæ Sp. B.) of the caudal feet
(uropoda Westw.), etc., to be a true Amphithoë[1] possesses it—that in many
species of Cerapus it is reduced to a scarcely perceptible
rudiment—nay, that it is sometimes present in youth and disappears
(although perhaps not without leaving some trace) at maturity, as was found by
Spence Bate to be the case in Acanthonotus Owenii and Atylus
carinatus, and I can affirm with regard to an Atylus of these seas,
remarkable for its plumose branchiæ—and that from all this, at the
present day when the increasing number of known Amphipoda and the splitting of
them into numerous genera thereby induced, compels us to descend to very minute
distinctive characters, we must nevertheless hesitate before employing the
secondary flagellum as a generic character. The case of Melita Fresnelii
therefore cannot excite any doubts as to Darwin’s theory.
CHAPTER III.
MORPHOLOGY OF CRUSTACEA—NAUPLIUS-LARVÆ.
If the absence of contradictions among the inferences deduced from them for a
narrow and consequently easily surveyed department must prepossess us in favour
of Darwin’s views, it must be welcomed as a positive triumph of his
theory if far-reaching conclusions founded upon it should subsequently
be confirmed by facts, the existence of which science, in its previous state,
by no means allowed us to suspect. From many results of this kind upon which I
could report, I select as examples, two, which were of particular importance to
me, and relate to discoveries the great significance of which in the morphology
and classification of the Crustacea will not be denied even by the opponents of
Darwin.
Considerations upon the developmental history of the Crustacea had led me to
the conclusion that, if the higher and lower Crustacea were at all derivable
from common progenitors, the former also must once have passed through
Nauplius-like conditions. Soon afterwards I discovered Naupliiform larvæ of
Shrimps (‘Archiv für Naturgeschichte’ 1860, i, p. 8), and I
must admit that this discovery gave me the first decided turn in Darwin’s
favour.
The similar number of segments[1] occurring in the Crabs and Macrura, Amphipoda
and Isopoda, in which the last seven segments are always different from the
preceding ones in the appendages with which they are furnished, could only be
regarded as an inheritance from the same ancestors. And if at the present day
the majority of the Crabs and Macrura, and indeed the Stalk-eyed Crustacea in
general, pass through Zoëa-like developmental states, and the same mode of
transformation was to be ascribed to their ancestors, the same thing must also
apply, if not to the immediate ancestors of the Amphipoda and Isopoda, at least
to the common progenitors of these and the Stalk-eyed Crustacea. Any such
assumption as this was, however, very hazardous, so long as not a single fact
properly relating to the Edriophthalma could be adduced in its support, as the
structure of this very coherent group seemed to be almost irreconcilable with
many peculiarities of the Zoëa. Thus, in my eyes, this point long
constituted one of the chief difficulties in the application of the Darwinian
views to the Crustacea, and I could scarcely venture to hope that I might yet
find traces of this passage through the Zoëa-form among the Amphipoda or
Isopoda, and thus obtain a positive proof of the correctness of this
conclusion. At this point Van Beneden’s statement that a cheliferous
Isopod (Tanais Dulongii), belonging, according to Milne-Edwards, to the
same family as the common Asellus aquaticus, possesses a carapace like
the Decapoda, directed my attention to these animals, and a careful examination
proved that these Isopods have preserved, more truly than any other adult
Crustacea, many of the most essential peculiarities of the Zoëae,
especially their mode of respiration. Whilst in all other Oniscoida the
abdominal feet serve for respiration, these in our cheliferous Isopod (Fig. 2)
are solely motory organs, into which no blood-corpuscle ever enters, and the
chief seat of respiration is, as in the Zoëae, in the lateral parts of
the carapace, which are abundantly traversed by currents of blood, and beneath
which a constant stream of water passes, maintained, as in Zoëae and the
adult Decapoda, by an appendage of the second pair of maxillæ, which is wanting
in all other Edriophthalma.
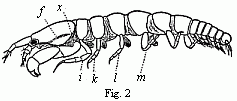
Fig. 2. Tanais dubius (?) Kr. hermaphrodite, magnified, showing the
orifice of entrance (x) into the cavity overarched by the carapace, in
which an appendage of the second pair of maxillæ (f) plays. On four feet
(i, k, l, m) are the rudiments of the lamellæ which subsequently form
the brood-cavity.
For both these discoveries, it may be remarked in passing, science is indebted
less to a happy chance than immediately to Darwin’s theory.
Species of Penëus live in the European seas, as well as here, and their
Nauplius-brood has no doubt repeatedly passed unnoticed through the
hands of the numerous naturalists who have investigated those seas, as well as
through my own,[2]
for it has nothing which could attract particular attention amongst the
multifarious and often wonderful Nauplius-forms. When I, fancying from
the similarity of its movements that it was a young Penëus-Zoëa, had for
the first time captured such a larva, and on bringing it under the microscope
found a Nauplius differing toto cœlo from this Zoëa,
I might have thrown it aside as being completely foreign to the developmental
series which I was tracing, if the idea of early Naupliiform stages of the
higher Crustacea, which indeed I did not believe to be still extant, had not at
the moment vividly occupied my attention.
And if I had not long been seeking among the Edriophthalma for traces of the
supposititious Zoëa-state, and seized with avidity upon everything that
promised to made this refractory Order serviceable to me, Van Beneden’s
short statement could hardly have affected me so much in the manner of an
electric shock, and impelled me to a renewed study of the Tanaides,
especially as I had once before plagued myself with them in the Baltic, without
getting any further than my predecessors, and I have not much taste for going
twice over the same ground.
CHAPTER IV.
SEXUAL PECULIARITIES AND DIMORPHISM.
Our Tanais, which in nearly all the particulars of its structure is an
extremely remarkable animal, furnished me with a second fact worthy of notice
in connection with the theory of the origin of species by natural selection.
When hand-like or cheliform structures occur in the Crustacea, these are
usually more strongly developed in the males than in the females, often
becoming enlarged in the former to quite a disproportionate size, as we have
already seen to be the case in Melita. A better known example of such
gigantic chelæ is presented by the males of the Calling Crabs (
Gelasimus ), which are said in running to carry these claws
“elevated, as if beckoning with them”—a statement which,
however, is not true of all the species, as a small and particularly
large-clawed one, which I have seen running about by thousands in the
cassava-fields at the mouth of the Cambriú, always holds them closely
pressed against its body.
A second peculiarity of the male Crustacea consists not unfrequently in a more
abundant development on the flagellum of the anterior antennæ of delicate
filaments which Spence Bate calls “auditory cilia,” and which I
have considered to be olfactory organs, as did Leydig before me, although I was
not aware of it. Thus they form long dense tufts in the males of many
Diastylidæ, as Van Beneden also states with regard to Bodotria, whilst
the females only possess them more sparingly. In the Copepoda, Claus called
attention to the difference of the sexes in this respect. It seems to me, as I
may remark in passing, that this stronger development in the males is greatly
in favour of the opinion maintained by Leydig and myself, as in other cases
male animals are not unfrequently guided by the scent in their pursuit of the
ardent females.
Now, in our Tanais, the young males up to the last change of skin
preceding sexual maturity resemble the females, but then they undergo an
important metamorphosis. Amongst other things they lose the moveable appendages
of the mouth even to those which serve for the maintenance of the respiratory
current; their intestine is always found empty, and they appear only to live
for love. But what is most remarkable is, that they now appear under two
different forms. Some (Fig. 3) acquire powerful, long-fingered, and very mobile
chelæ, and, instead of the single olfactory filament of the female, have from
12 to 17 of these organs, which stand two or three together on each joint of
the flagellum. The others (Fig. 5) retain the short thick form of the chelæ of
the females; but, on the other hand, their antennæ (Fig. 6) are equipped with a
far greater number of olfactory filaments, which stand in groups of from five
to seven together.

Fig. 3. Head of the ordinary form of the male of Tanais dubius (?) Kr.
magnified. The terminal setæ of the second pair of antennæ project between the
cheliferous feet.
Fig. 4. Buccal region of the same from below; lambda, labrum.
Fig. 5. Head of the rarer form of the male, magnified.
Fig. 6. Flagellum of the same, with olfactory filaments, magnified.
In the first place, and before inquiring into its significance, I will say a
word upon this fact itself. It was natural to consider whether two different
species with very similar females and very different males might not perhaps
live together, or whether the males, instead of occurring in two sharply
defined forms, might not be only variable within very wide limits. I can admit
neither of these suppositions. Our Tanais lives among densely interwoven
Confervæ, which form a coat of about an inch in thickness upon stones in the
neighbourhood of the shore. If a handful of this green felt is put into a large
glass with clear sea-water, the walls of the glass are soon seen covered with
hundreds, nay with thousands, of these little, plump, whitish Isopods. In this
way I have examined thousands of them with the simple lens, and I have also
examined many hundreds with the microscope, without finding any differences
among the females, or any intermediate forms between the two kinds of males.
To the old school this occurrence of two kinds of males will appear to be
merely a matter of curiosity. To those who regard the “plan of
creation” as the “free conception of an Almighty intellect, matured
in the thoughts of the latter before it is manifested in palpable, external
forms,” it will appear to be a mere caprice of the Creator, as it
is inexplicable either from the point of view of practical adaptation, or from
the “typical plan of structure.” From the side of Darwin’s
theory, on the contrary, this fact acquires meaning and significance, and it
appears in return to be fitted to throw light upon a question in which Bronn
saw “the first and most material objection against the new theory,”
namely, how it is possible that from the accumulation in various directions of
the smallest variations running out of one another, varieties and species are
produced, which stand out from the primary form clearly and sharply like the
petiolated leaf of a Dicotyledon, and are not amalgamated with the primary form
and with each other like the irregular curled lobes of a foliaceous Lichen.
Let us suppose that the males of our Tanais, hitherto identical in
structure, begin to vary, in all directions as Bronn thinks, for aught I care.
If the species was adapted to its conditions of existence, if the best
in this respect had been attained and secured by natural selection, fresh
variations affecting the species as a species would be retrogressions, and thus
could have no prospect of prevailing. They must rather have disappeared again
as they arose, and the lists would remain open to the males under variation,
only in respect of their sexual relations. In these they might acquire
advantages over their rivals by their being enabled either to seek or to seize
the females better. The best smellers would overcome all that were inferior to
them in this respect, unless the latter had other advantages, such as more
powerful chelæ, to oppose to them. The best claspers would overcome all less
strongly armed champions, unless these opposed to them some other advantage,
such as sharper senses. It will be easily understood how in this manner all the
intermediate steps less favoured in the development of the olfactory filaments
or of the chelæ would disappear from the lists, and two sharply defined forms,
the best smellers and the best claspers, would remain as the sole adversaries.
At the present day the contest seems to have been decided in favour of the
latter, as they occur in greatly preponderating numbers, perhaps a hundred of
them to one smeller.
To return to Bronn’s objection. When he says that “for the support
of the Darwinian theory, and in order to explain why many species do not
coalesce by means of intermediate forms, he would gladly discover some external
or internal principle which should compel the variations of each species to
advance in one direction, instead of merely permitting them in all
directions,” we may, in this as in many other cases, find such a
principle in the fact that actually only a few directions stand open in which
the variations are at the same time improvements, and in which therefore they
can accumulate and become fixed; whilst in all others, being either indifferent
or injurious, they will go as lightly as they come.
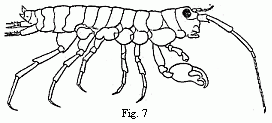
Fig. 7. Orchestia Darwinii, n. sp. male.
The occurrence of two kinds of males in the same species may perhaps not be a
very rare phenomenon in animals in which the males differ widely from the
females in structure. But only in those which can be procured in sufficient
abundance, will it be possible to arrive at a conviction that we have not
before us either two different species, or animals of different ages. From my
own observation, although not very extensive, I can give a second example. It
relates to a shore-hopper ( Orchestia ). The animal (Fig. 7) lives in
marshy places in the vicinity of the sea, under decaying leaves, in the loose
earth which the Marsh Crabs ( Gelasimus, Sesarma, Cyclograpsus, etc.)
throw up around the entrance to their borrows, and even under dry cow-dung and
horse-dung. If this species removes to a greater distance from the shore than
the majority of its congeners (although some of them advance very far into the
land and even upon mountains of a thousand feet in height, such as O.
tahitensis, telluris, and sylvicola ), its male differs still more
from all known species by the powerful chelæ of the second pair of feet.
Orchestia gryphus, from the sandy coast of Monchgut, alone presents a
somewhat similar structure, but in a far less degree; elsewhere the form of the
hand usual in the Amphipoda occurs. Now there is a considerable difference
between the males of this species, especially in the structure of these
chelæ—a different so great that we can scarcely find a parallel to it
elsewhere between two species of the genus—and yet, as in Tanais,
we do not meet with a long series of structures running into one another, but
only two forms united by no intermediate terms (Figs. 8 and 9). The males would
be unhesitatingly regarded as belonging to two well-marked species if they did
not live on the same spot, with undistinguishable females. That the two forms
of the chelæ of the males occur in this species is so far worthy of notice,
because the formation of the chelæ, which differs widely from the ordinary
structure in the other species, indicates that it has quite recently undergone
considerable changes, and therefore such a phenomenon was to be expected in it
rather than in other species.
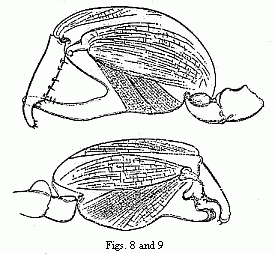
Figs. 8 and 9. The two forms of the chelæ of the male of Orchestia
Darwinii, magnified.
I cannot refrain from taking this opportunity of remarking that (so far as
appears from Spence Bate’s catalogue), for two different kinds of males (
Orchestia telluris and sylvicola ) which live together in the
forests of New Zealand, only one form of female is known, and hazarding the
supposition that we have here a similar case. It does not seem to me to be
probable that two nearly allied species of these social Amphipoda should occur
mixed together under the same conditions of life.
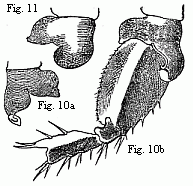
Fig. 10. Coxal lamella of the penultimate pair of feet of the male ( a
), and coxal lamella, with the three following joints of the same pair of feet
of the female ( b ) of Melita Messalina, magnified.
Fig.
11. Coxal lamella of the same pair of feet of the female of M.
insatiabilis.
As the males of several species of Melita are distinguished by the powerful
unpaired clasp-forceps, the females of some other species of the same genus are
equally distinguished from all other Amphipoda by the circumstance that in them
a peculiar apparatus is developed which facilitates their being held by the
male. The coxal lamellæ of the penultimate pair of feet are produced into
hook-like processes, of which the male lays hold with the hands of the first
pair of feet. The two species in which I am acquainted with this structure are
amongst the most salacious animals of their order, even females which are laden
with eggs in all stages of development, not unfrequently have their males upon
their backs. The two species are nearly allied to Melita palmata Leach (
Gammarus Dugesii, Edw.), which is widely distributed on the European
coasts, and has been frequently investigated; unfortunately, however, I can
find no information as to whether the females of this or any other European
species possess a similar contrivance. In M. exilii all the coxal
lamellæ are of the ordinary formation. Nevertheless, be this as it will,
whether they exist in two or in twenty species, the occurrence of these
peculiar hook-like processes is certainly very limited.
Now our two species live sheltered beneath slightly tilted stones in the
neighbourhood of the shore: one of them, Melita Messalina, so high that
it is but rarely covered by the water; the other, Melita insatiabilis, a
little lower; both species live together in numerous swarms. We cannot
therefore suppose that the loving couples are threatened with disturbance more
frequently than those of other species, nor would it be more difficult for the
male, than for those of other species, in case of his losing his female, to
find a new one. Nor is it any more easy to see how the contrivance on the body
of the female for insuring the act of copulation could be injurious to other
species. But so long as it is not demonstrated that our species are
particularly in want of this contrivance, or that the latter would rather be
injurious than beneficial to other species, its presence only in these few
Amphipoda will have to be regarded not as the work of far-seeing wisdom, but as
that of a favourable chance made use of by Natural Selection. Under the latter
supposition its isolated occurrence is intelligible, whilst we cannot perceive
why the Creator blessed just these few species with an apparatus which he found
to be quite compatible with the “general plan of structure” of the
Amphipoda, and yet denied it to others which live under the same external
conditions, and equal them even in their extraordinary salacity. Associated
with, or in the immediate vicinity of the two species of Melita, live
two species of Allorchestes, the pairs of which are met with almost more
numerously than the single animals, and yet their females show no trace of the
above-mentioned processes of the coxal lamellæ.
These cases, I think, must be brought to bear against the conception supported
with so much genius and knowledge by Agassiz, that species are embodied
thoughts of the Creator; and, with these, all similar instances in which
arrangements which would be equally beneficial to all the species of a group
are wanting in the majority and only conferred upon a few special favourites,
which do not seem to want them any more than the rest.
CHAPTER V.
RESPIRATION IN LAND CRABS.
Among the numerous facts in the natural history of
the Crustacea upon which a new and clear light is thrown by
Darwin’s theory, besides the two forms of the males in our
Tanais and in Orchestia Darwinii, there is one which
appears to me of particular importance, namely, the character of
the branchial cavity in the air-breathing Crabs, of which,
unfortunately, I have been unable to investigate some of the most
remarkable (Gecarcinus, Ranina). As this character, namely,
the existence of an entrance behind the branchiæ, has
hitherto been noticed, even as a fact, only in Ranina, I
will go into it in some detail. I have already mentioned that, as
indeed is required by Darwin’s theory, this entrant orifice
is produced in different manners in the different families.
In the Frog-crab (Ranina) of the Indian Ocean, which,
according to Rumphius, loves to climb up on the roofs of the
houses, the ordinary anterior entrant orifice is entirely wanting
according to Milne-Edwards, and the entrance of a canal opening
into the hindmost parts of the branchial cavity is situated beneath
the commencement of the abdomen.
The case is most simple in some of the Grapsoidæ, as in
Aratus Pisonii, a charming, lively Crab which ascends the
mangrove bushes (Rhizophora) and gnaws their leaves. By
means of its short but remarkably acute claws, which prick like
pins when it runs over the hand, this Crab climbs with the greatest
agility upon the thinnest twigs. Once, when I had one of these
animals sitting upon my hand, I noticed that it elevated the hinder
part of its carapace, and that by this means a wide fissure was
opened upon each side above the last pair of feet, through which I
could look far into the branchial cavity. I have since been unable
to procure this remarkable animal again, but on the other hand, I
have frequently repeated the same observation upon another animal
of the same family (apparently a true Grapsus), which lives
abundantly upon the rocks of our coast. Whilst the hinder part of
the carapace rises and the above-mentioned fissure is formed, the
anterior part seems to sink, and to narrow or entirely close the
anterior entrant orifice. Under water the elevation of the carapace
never takes place. The animal therefore opens its branchial cavity
in front or behind, according as it has to breathe water or air.
How the elevation of the carapace is effected I do not know, but I
believe that a membranous sac, which extends from the body cavity
far into the branchial cavity beneath the hinder part of the
carapace, is inflated by the impulsion of the fluids of the body,
and the carapace is thereby raised.
I have also observed the same elevation of the carapace in some
species of the allied genera Sesarma and
Cyclograpsus, which dig deep holes in marshy ground, and often
run about upon the wet mud, or sit, as if keeping watch, before
their burrows. One must, however, wait for a long time with these
animals, when taken out of the water, before they open their
branchial cavity to the air, for they possess a wonderful
arrangement, by means of which they can continue to breathe water
for some time when out of the water. The orifices for the egress of
the water which has served for respiration, are situated in these,
as in most Crabs, in the anterior angles of the buccal frame
(“cadre buccal,” M.-Edw.), whilst the entrant fissures
of the branchial cavity extend from its hinder angles above the
first pair of feet. Now that portion of the carapace which extends
at the sides of the mouth between the two orifices
(“régions ptérygostomiennes”), appears in
our animals to be divided into small square compartments.
Milne-Edwards has already pointed this out as a particularly
remarkable peculiarity. This appearance is caused partly by small
wart-like elevations, and partly and especially by curious
geniculated hairs, which to a certain extent constitute a fine net
or hair-sieve extended immediately over the surface of the
carapace. Thus when a wave of water escapes from the branchial
cavity, it immediately becomes diffused in this network of hairs
and then again conveyed back to the branchial cavity by vigorous
movements of the appendage of the outer maxilliped which works in
the entrant fissure. Whilst the water glides in this way over the
carapace in the form of a thin film, it will again saturate itself
with oxygen, and may then serve afresh for the purposes of
respiration. In order to complete this arrangement the outer
maxillipeds, as indeed has long been known, bear a projecting ridge
furnished with a dense fringe of hairs, which commences in front
near their median line and passes backwards and outwards to the
hinder angle of the buccal frame. Thus the two ridges of the right
and left sides form together a triangle with the apex turned
forwards,—a breakwater by which the water flowing from the
branchial cavity is kept away from the mouth and reconducted to the
branchial cavity. In very moist air the store of water contained in
the branchial cavity may hold out for hours, and it is only when
this is used up that the animal elevates its carapace in order to
allow the air to have access to its branchiæ from behind.
In Eriphia gonagra the entrant orifices of the
respiratory cavity serving for aerial respiration are situated,
not, as in the Grapsoidæ, above, but behind the last pair of
feet at the sides of the abdomen.
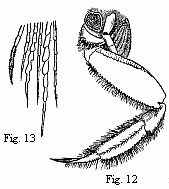
Fig. 12. Posterior entrance to the branchial cavity of
Ocypoda rhombea, Fab. The carapace and the fourth foot of the right side
are removed.
Fig. 13. Points of some of the hairs of the basal joints of
the foot, magnified.
The swift-footed Sand-Crabs (Ocypoda) are exclusively terrestrial
animals, and can scarcely live for a single day in water; in a much shorter
period a state of complete relaxation occurs and all voluntary movements
cease.[1] In these a
peculiar arrangement on the feet of the third and fourth pairs (Fig. 12) has
long been known, although its connexion with the branchial cavity has not been
suspected. These two pairs of feet are more closely approximated than the rest;
the opposed surfaces of their basal joints (therefore the hinder surface on the
third, and the anterior surface on the fourth feet) are smooth and polished,
and their margins bear a dense border of long, silky, and peculiarly formed
hairs (Fig. 13). Milne-Edwards who rightly compares these surfaces, as to their
appearance, with articular surfaces, thinks that they serve to diminish the
friction between the two feet. In considering this interpretation, the question
could not but arise why such an arrangement for the diminution of friction
should be necessary in these particular Crabs and between these two feet,
leaving out of consideration the fact that the remarkable brushes of hair,
which on the other hand must increase friction, also remain unexplained. But as
I was bending the feet of a large Sand-Crab to and fro in various directions,
in order to see in what movements of the animal friction occurred at the place
indicated, and whether these might, perhaps, be movements of particular
importance to it and such as would frequently recur, I noticed, when I had
stretched the feet widely apart, in the hollow between them a round orifice of
considerable size, through which air could easily be blown into the branchial
cavity, and a fine rod might even be introduced into it. The orifice opens into
the branchial cavity behind a conical lobe, which stands above the third foot
in place of a branchia which is wanting in Ocypoda. It is bounded
laterally by ridges, which rise above the articulation of the foot, and to
which the lower margin of the carapace is applied. Exteriorly, also, it is
overarched by these ridges with the exception of a narrow fissure. This fissure
is overlaid by the carapace, which exactly at this part projects further
downwards than elsewhere, and in this way a complete tube is formed. Whilst in
Grapsus the water is allowed to reach the branchiæ only from the front,
I saw it in Ocypoda flow in also through the orifice just described.
In the position of posterior entrant orifice and the accompanying peculiarities
of the third and fourth pairs of feet, two other non-aquatic species of the
same family, which I have had the opportunity of examining, agree with
Ocypoda. One of these, perhaps Gelasimus vocans, which lives in
the mangrove swamps, and likes to furnish the mouth of its burrow with a thick,
cylindrical chimney of several inches in height, has the brushes on the basal
joints of the feet in question composed of ordinary hairs. The other, a smaller
Gelasimus, not described in Milne-Edwards’ ‘Natural History
of Crustacea,’ which prefers drier places and is not afraid to run about
on the burning sand under the vertical rays of the noonday sun in December, but
can also endure being in water at least for several weeks, resembles
Ocypoda in having these brushes composed of non-setiform, delicate
hairs, indeed even more delicate and more regularly constructed than in
Ocypoda.[2]
What may be the significance of these peculiar hairs,—whether they only
keep foreign bodies from the branchial cavity,—whether they furnish
moisture to the air flowing past them,—or whether, as their aspect,
especially in the small Gelasimus, reminds one of the olfactory
filaments of the Crabs, they may also perform similar functions,—are
questions the due discussion of which would lead us too far from our subject.
Nevertheless it may be remarked that in both species, especially in
Ocypoda, the olfactory filaments in their ordinary situation are very
much reduced, and when they are in the water their flagella never perform the
peculiar beating movements which may be observed in other Crabs, and even in
the larger Gelasimus; moreover, the organ of smell must probably be
sought in these air-breathing Crabs, as in the air-breathing Vertebrata, at the
entrance to the respiratory cavity.
So much for the facts with regard to the aerial respiration of
the Crabs. It has already been indicated why Darwin’s theory
requires that when any peculiar arrangements exist for aerial
respiration, these will be differently constructed in different
families. That experience is in perfect accordance with this
requirement is the more in favour of Darwin, because the schoolmen
far from being able to foresee or explain such profound
differences, must rather regard them as extremely surprising. If,
in the nearly allied families of the Ocypodidæ and
Grapsoidæ, the closest agreement prevails in all the
essential conditions of their structure; if the same plan of
structure is slavishly followed in everything else, in the organs
of sense, in the articulation of the limbs, in every trabecula and
tuft of hairs in the complicated framework of the stomach, and in
all the arrangements subserving aquatic respiration, even to the
hairs of the flagella employed in cleaning the
branchiæ,—why have we suddenly this exception, this
complete difference, in connection with aerial respiration?
The schoolmen will scarcely have an answer for this question,
except by placing themselves on the theologico-teleological
stand-point which has justly fallen into disfavour amongst us, and
from which the mode of production of an arrangement is supposed to
be explained, if its “adaptation” to the animal can be
demonstrated. From this point of view we might certainly say that a
widely gaping fissure which had nothing prejudicial in it to
Aratus Pisonii among the foliage of the mangrove bushes, was
not suitable to the Ocypoda living in sand; that in the
latter, in order to prevent the penetration of the sand, the
orifice of the branchial cavity must be placed at its lowest part,
directed downwards, and concealed between broad surfaces fringed
with protective brushes of hair. It is far from the intention of
these pages to enter upon a general refutation of this theory of
adaptation. Indeed there is scarcely anything essential to be added
to the many admirable remarks that have been made upon this subject
since the time of Spinoza. But this may be remarked, that I regard
it as one of the most important services of the Darwinian theory
that it has deprived those considerations of usefulness which are
still undeniable in the domain of life, of their mystical
supremacy. In the case before us it is sufficient to refer to the
Gelasimus of the mangrove swamps, which shares the same conditions
of life with various Grapsoidæ and yet does not agree with
them, but with the arenicolous Ocypoda.
CHAPTER VI.
STRUCTURE OF THE HEART IN THE EDRIOPHTHALMA.
Scarcely less striking than the example of the air-breathing Crabs, is the
behaviour of the heart in the great section Edriophthalma, which may
advantageously be divided, after the example of Dana and Spence Bate, only into
two orders, the Amphipoda and the Isopoda.
In the Amphipoda, to which the above-mentioned naturalists correctly refer the
Caprellidæ and Cyamidæ (Latreille’s Læmodipoda), the heart has
always the same position; it extends in the form of a long tube through the six
segments following the head, and has three pairs of fissures, furnished with
valves, for the entrance of the blood, situated in the second, third, and
fourth of these segments. It was found to be of this structure by La Valette in
Niphargus (Gammarus puteanus), and by Claus in Phronima;
and I have found it to be the same in a considerable number of species
belonging to the most different families.[1]
The sole unimportant exception which I have hitherto met with is presented by
the genus Brachyscelus,[2] in which the heart possesses only two pairs of
fissures, as it extends forward only into the second body-segment, and is
destitute of the pair of fissures situated in this segment in other forms.[3]
Considering this uniformity presented by the heart in the entire order of the
Amphipoda, it cannot but seem very remarkable, that in the very next order of
the Isopoda, we find it to be one of the most changeable organs.
In the cheliferous Isopods (Tanais) the heart resembles that of the
Amphipoda in its elongated tubular form, as well as in the number and position
of the fissures, but with this difference, that the two fissures of each pair
do not lie directly opposite each other.
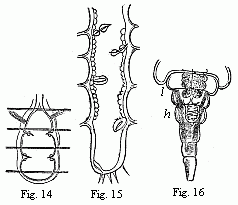
Fig. 14. Heart of a young Cassidina. Fig. 15. Heart
of a young Anilocra.
Fig. 16. Abdomen of the male of Entoniscus
Cancrorum. h. Heart. l. Liver.
In all other Isopoda the heart is removed towards the abdomen. In the
wonderfully deformed parasitic Isopods of the Porcellanæ (Entoniscus
porcellanæ), the spherical heart of the female is confined to a short space
of the elongated first abdominal segment, and seems to possess only a single
pair of fissures. In the male of Entoniscus Cancrorum (n. sp.), the
heart (Fig. 16) is situated in the third abdominal segment. In the
Cassidinæ, the heart (Fig. 14) is likewise short and furnished with two
pairs of fissures, situated in the last segment of the thorax and the first
segment of the abdomen. Lastly, in a young Anilocra, I find the heart
(Fig. 15) extending through the whole length of the abdomen and furnished with
four (or five?) fissures, which are not placed in pairs but alternately to the
right and left in successive segments. In other animals of this order, which I
have as yet only cursorily examined, further differences will no doubt occur.
But why, in two orders so nearly allied to each other, should we find in the
one such a constancy, in the other such a variability, of the same highly
important organ? From the schoolmen we need expect no explanation, they will
either decline the discussion of the “wherefore” as foreign to
their province, as lying beyond the boundaries of Natural History, or seek to
put down the importunate question by means of a sounding paraphrase of the
facts, abundantly sprinkled with Greek words. As I have unfortunately forgotten
my Greek, the second way out of the difficulty is closed to me; but as I
luckily reckon myself not amongst the incorporated masters, but, to use Baron
von Liebig’s expression, amongst the “promenaders on the outskirts
of Natural History,” this affected hesitation of the schoolmen cannot
dissuade me from seeking an answer, which indeed presents itself most naturally
from Darwin’s point of view.
As not only the Tanaides (which reasons elsewhere stated (vide
suprà) justify us in regarding as particularly nearly related to the
primitive Isopod) and the Amphipoda, but also the Decapod Crustacea, possess a
heart with three pairs of fissures essentially in the same position; and as the
same position of the heart recurs (vide infrà) even in the
embryos of the Mantis-Shrimps (Squilla), in which the heart of the adult
animal, and even, as I have elsewhere shown, that of the larvæ when still far
from maturity, extends in the form of a long tube with numerous openings far
into the abdomen, we must unhesitatingly regard the heart of the Amphipoda as
the primitive form of that organ in the Edriophthalma. As, moreover, in these
animals the blood flows from the respiratory organs to the heart without
vessels, it is very easy to see how advantageous it must be to them to have
these organs as much approximated as possible. We have reason to regard as the
primitive mode of respiration, that occurring in Tanais (vide
suprà). Now, where, as in the majority of the Isopoda, branchiæ were
developed upon the abdomen, the position and structure of the heart underwent a
change, as it approached them more nearly, but without the reproduction of a
common plan for these earlier modes of structure, either because this
transformation of the heart took place only after the division of the primary
form into subordinate groups, or because, at least at the time of this
division, the varying heart had not yet become fixed in any new form. Where, on
the contrary, respiration remained with the anterior part of the
body,—whether in the primitive fashion of Zoëa, as in the
Tanaides, or by the development of branchiæ on the thorax, as in the
Amphipoda,—the primitive form of the heart was inherited unchanged,
because any variations which might make their appearance were rather injurious
than advantageous, and disappeared again immediately.
I close this series of isolated examples with an observation which indeed only
half belongs to the province of the Crustacea to which these pages ought to be
confined, and which also has no further connexion with the preceding
circumstances than that of being an “intelligible and
intelligence-bringing fact” only from the point of view of Darwin’s
theory. To-day as I was opening a specimen of Lepas anatifera in order
to compare the animal with the description in Darwin’s ‘Monograph
on the Subclass Cirripedia,’ I found in the shell of this Cirripede, a
blood-red Annelide, with a short, flat body, about half an inch long and two
lines in breadth, with twenty-five body-segments, and without projecting
setigerous tubercles or jointed cirri. The small cephalic lobe bore four eyes
and five tentacles; each body-segment had on each side at the margin a tuft of
simple setæ directed obliquely upwards, and at some distance from this, upon
the ventral surface, a group of thicker setæ with a strongly uncinate bidentate
apex. There was above each of the lateral tufts of bristles a branchia,
simple on a few of the foremost segments, and then strongly arborescent to the
end of the body. The animal, a female filled with ova, evidently, from these
characters, belongs to the family of the Amphinomidæ; the only family the
members of which, being excellent swimmers, live in the open sea.
That this animal had not strayed accidentally into the Lepas, but
appertained to it as a regular and permanent guest, is evidenced by its
considerable size in proportion to the narrow entrance of the test of the
Lepas, by the complete absence of the iridescence which usually
distinguishes the skin of free Annelides and especially of the Amphinomidæ, by
the formation and position of the inferior setæ, etc. But that a worm belonging
to this particular family Amphinomidæ living in the high sea, occurs as a guest
in the Lepas, which also floats in the sea attached to wood, etc., is at
once intelligible from the stand-point of the Darwinian theory, whilst the
relationship of this parasite to the free-living worms of the open sea remains
perfectly unintelligible under the supposition that it was independently
created for dwelling in the Lepas.
But however favourable the examples hitherto referred to may be for Darwin, the
objection may be raised against them, and that with perfect justice, that they
are only isolated facts, which, when the considerations founded upon them are
carried far beyond what is immediately given, may only too easily lead us from
the right path, with the deceptive glimmer of an ignis fatuus. The
higher the structure to be raised, the wider must be the assuring base of
well-sifted facts.
Let us turn then to a wider field, that of the developmental history of the
Crustacea, upon which science has already brought together a varied abundance
of remarkable facts, which, however, have remained a barren accumulation of
unmanageable raw-material, and let us see how, under Darwin’s hand, these
scattered stones unite to form a well-jointed structure, in which everything,
bearing and being borne, finds its significant place. Under Darwin’s
hand! for I shall have nothing to do except just to place the building stones
in the position which his theory indicates for them. “When kings build,
the carters have to work.”
CHAPTER VII.
STRUCTURE OF THE HEART IN THE EDRIOPHTHALMA.
Let us first glance over the extant facts.
Among the Stalk-eyed Crustacea (Podophthalma) we know only a very few
species which quit the egg in the form of their parents, with the full number
of well-jointed appendages to the body. This is the case according to Rathke[1] in the European
fresh-water Crayfish, and according to Westwood in a West Indian Land Crab
(Gecarcinus). Both exceptions therefore belong to the small number of
Stalk-eyed Crustacea which live in fresh water or on the land, as indeed in
many other cases fresh-water and terrestrial animals undergo no
transformations, whilst their allies in the sea have a metamorphosis to
undergo. I may refer to the Earthworms and Leeches among the Annelida, which
chiefly belong to the land and to fresh water,—to the Planariæ of
the fresh waters and the Tetrastemma of the sparingly saline Baltic
among the Turbellaria,—to the Pulmonate Gasteropoda, and to the
Branchiferous Gasteropoda of the fresh waters, the young of which (according to
Troschel’s ‘Handb. der Zoologie’) have no ciliated buccal
lobes, although such organs are possessed by the very similar Periwinkles
(Littorina).
All the marine forms of this section appear to be subject to a
more or less considerable metamorphosis. This appears to be only
inconsiderable in the common Lobster, the young of which, according
to Van Beneden, are distinguished from the adult animal, by having
their feet furnished, like those of Mysis, with a swimming
branch projecting freely outwards. From a figure given by Couch the
appendages of the abdomen and tail also appear to be wanting.
Far more profound is the difference of the youngest brood from the sexually
mature animal in by far the greater majority of the Podophthalma, which quit
the egg in the form of Zoëa. This young form occurs, so far as our
present observations go, in all the Crabs, with the sole exception of the
single species investigated by Westwood. I say species, and not
genus, for in the same genus, Gecarcinus, Vaughan Thompson found
Zoëa-brood,[2]
which is also met with in other terrestrial Crabs (Ocypoda, Gelasimus,
etc.). All the Anomura seem likewise to commence their lives as Zoëæ: witness
the Porcellanæ, the Tatuira (Hippa emerita) and the Hermit
Crabs. Among the Macrura we are acquainted with the same earliest form
principally in several Shrimps and Prawns, such as Crangon (Du Cane),
Caridina (Joly), Hippolyte, Palæmon, Alpheus, etc. Lastly, it is
not improbable, that the youngest brood of the Mantis-Shrimps (Squilla)
is also in the same case.
The most important peculiarities which distinguish this
Zoëa-brood from the adult animal, are as follows:—
The middle-body with its appendages, those five pairs of feet to
which these animals owe their name of Decapoda, is either entirely
wanting, or scarcely indicated; the abdomen and tail are destitute
of appendages, and the latter consists of a single piece. The
mandibles, as in the Insecta, have no palpi. The maxillipedes, of
which the third pair is often still wanting, are not yet brought
into the service of the mouth, but appear in the form of biramose
natatory feet. Branchiæ are wanting, or where their first
rudiments may be detected as small verruciform prominences, these
are dense cell-masses, through which the blood does not yet flow,
and which therefore have nothing to do with respiration. An
interchange of the gases of the water and blood may occur all over
the thin-skinned surface of the body; but the lateral parts of the
carapace may unhesitatingly be indicated as the chief seat of
respiration. They consist, exactly as described by Leydig in the
Daphniæ, of an outer and inner lamina, the space
between which is traversed by numerous transverse partitions
dilated at their ends; the spaces between these partitions are
penetrated by a more abundant flow of blood than occurs anywhere
else in the body of the Zoëa. To this may be added that a
constant current of fresh water passes beneath the carapace in a
direction from behind forwards, maintained as in the adult animal,
by a foliaceous or linguiform appendage of the second pair of
maxillæ (Fig. 18). The addition of fine coloured particles to
the water allows this current of water to be easily detected even
in small Zoëæ.
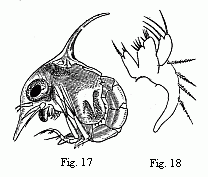
Fig. 17. Zoëa of a Marsh Crab (Cyclograpsus ?),
magnified.
Fig. 18. Maxilla of the second pair in the same species,
magnified.
The Zoëæ of the Crabs (Fig. 17) are usually distinguished by long, spiniform
processes of the carapace. One of these projects upwards from the middle of the
back, a second downwards from the forehead, and frequently there is a shorter
one on each side near the posterior inferior angles of the carapace. All these
processes are, however, wanting in Maia according to Couch, and in
Eurynome according to Kinahan; and in a third species of the same group
of the Oxyrhynchi (belonging or nearly allied to the genus
Achæus) I also find only an inconsiderable dorsal spine, whilst the
forehead and sides are unarmed. This is another example warning us to be
cautious in deductions from analogy. Nothing seemed more probable than to refer
back the beak-like formation of the forehead in the Oxyrhynchi to the frontal
process of the Zoëa, and now it appears that the young of the Oxyrhynchi are
really quite destitute of any such process. The following are more important
peculiarities of the Zoëæ of the Crabs, although less striking than these
processes of the carapace which, in combination with the large eyes, often give
them so singular an appearance:—the anterior (inner) antennæ are simple,
not jointed, and furnished at the extremity with from two to three olfactory
filaments; the posterior (outer) antennæ frequently run out into a remarkably
long spine-like process (“styliform process,” Spence Bate), and
bear, on the outside, an appendage, which is sometimes very minute
(“squamiform process” of Spence Bate), corresponding with the
antennal scale of the Prawns,[3] and the first rudiment of the future flagellum
is often already recognisable. Of natatory feet (afterwards maxillipeds) only
two pairs are present; the third (not, as Spence Bate thinks, the first) is
entirely wanting, or, like the five following pairs of feet, present only as a
minute bud. The tail, of very variable form, always bears three pairs of
setæ at its hinder margin. The Zoëæ of the Crabs usually maintain themselves in
the water in such a manner that the dorsal spine stands upwards, the abdomen is
bent forwards, the inner branch of the natatory feet is directed forwards, and
the outer one outwards and upwards.
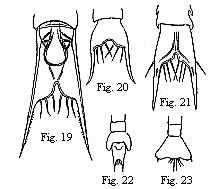
Figs. 19 to 23. Tails of the Zoëæ of various Crabs.
Fig. 19. Pinnotheres. Fig. 20. Sesarma. Fig. 21. Xantho.
Figs.
22 and 23 of unknown origin.
It is further to be remarked that the Zoëæ of the
Crabs, as also of the Porcellanæ, of the Tatuira and
of the Shrimps and Prawns, are enveloped, on escaping from the egg,
by a membrane veiling the spinous processes of the carapace, the
setæ of the feet, and the antennæ, and that they cast
this in a few hours. In Achæus I have observed that
the tail of this earliest larval skin resembles that of the
larvæ of Shrimps and Prawns, and the same appears to be the
case in Maia (see Bell, ‘Brit. Stalk-eyed
Crust.’ p. 44). Widely as they seem to differ from them at
the first glance, the Zoëæ of the
Porcellanæ (Fig. 24) approach those of the true Crabs
very closely. The antennæ, organs of the mouth, and natatory
feet, exhibit the same structure. But the tail bears five
pairs of setæ, and the dorsal spine is wanting, whilst, on
the contrary, the frontal process and the lateral spines are of
extraordinary length, and directed straight forward and
backward.
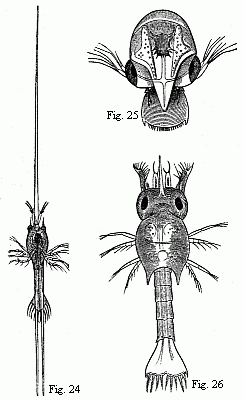
Fig. 24. Zoëa of Porcellana stellicola,
F. Müll., magnified.
Fig. 25. Zoëa of the Tatuira (Hippa emerita), magnified.
Fig. 26. Zoëa of a small Hermit Crab, magnified.
The Zoëa of the Tatuira (Fig. 25) also appears to differ
but little from those of the true Crabs, which it likewise
resembles in its mode of locomotion. The carapace possesses only a
short, broad frontal process; the posterior margin of the tail is
edged with numerous short setæ.
The Zoëa of the Hermit Crabs (Fig. 26) possesses the simple
inner antennæ of the Zoëa of the true Crabs; the outer
antennæ bear upon the outside on a short stalk a lamella of
considerable size analogous to the scale of the antennæ of
the Prawns; on the inside, a short, spine-like process; and between
the two the flagellum, still short, but already furnished with two
apical setæ. As in the Crabs, there are only two pairs of
well-developed natatory feet (maxillipedes), but the third pair is
also present in the form of a two-jointed stump of considerable
size, although still destitute of setæ. The tail bears five
pairs of setæ. The little animal usually holds itself
extended straight in the water, with the head directed
downwards.
This is also the position in which we usually see the
Zoëæ of the Shrimps and Prawns (Fig. 27), which agree in
their general appearance with those of the Hermit Crabs. Between
the large compound eyes there is in them a small median eye. The
inner antennæ bear, at the end of a basal joint sometimes of
considerable length, on the inside a plumose seta, which also
occurs in the Hermit Crabs, and on the outside a short terminal
joint with one or more olfactory filaments. The outer antennæ
exhibit a well-developed and sometimes distinctly articulated
scale, and within this usually a spiniform process; the flagellum
appears generally to be still wanting. The third pair of
maxillipedes seems to be always present, at least in the form of
considerable rudiments. The spatuliform caudal lamina bears from
five to six pairs of setæ on its hinder margin.
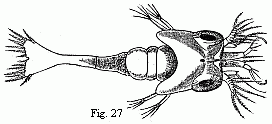
Fig. 27. Zoëa of a Palæmon residing upon
Rhizostoma cruciatum, Less., magnified.
The development of the Zoëa-brood to the sexually mature animal was traced by
Spence Bate in Carcinus mænas. He proved that the metamorphosis is a
perfectly gradual one, and that no sharply separated stages of development,
like the caterpillar and pupa of the Lepidoptera, could be defined in it.
Unfortunately we possess only this single complete series of observations, and
its results cannot be regarded at once as universally applicable; thus the
young Hermit Crabs retain the general aspect and mode of locomotion of Zoëæ,
whilst the rudiments of the thoracic and abdominal feet are growing, and then,
when these come into action, appear at once in a perfectly new form, which
differs from that of the adult animal chiefly by the complete symmetry of the
body and by the presence of four pairs of well-developed natatory feet on the
abdomen.[4]
The development of the Palinuridiæ seems to be very
peculiar. Claus found in the ova of the Spiny Lobster
(Palinurus), embryos with a completely segmented body, but
wanting the appendages of the tail, abdomen, and last two segments
of the middle-body; they possess a single median and considerably
compound eye; the anterior antennæ are simple, the posterior
furnished with a small secondary branch; the mandibles have no
palpi; the maxillipedes of the third pair, like the two following
pairs of feet, are divided into two branches of nearly equal
length; whilst the last of the existing pairs of feet and the
second pair of maxillipedes bear only an inconsiderable secondary
branch. Coste, as is well known, asserts that he has bred young
Phyllosomata from the ova of this lobster—a statement
that requires further proof, especially as the more recent
investigations of Claus upon Phyllosoma by no means appear
to be in its favour.
The large compound eyes, which usually soon become moveable, and
sometimes stand upon long stalks even in the earliest period, as
well as the carapace, which covers the entire fore-body, indicate
at once that the position of the larvæ hitherto considered,
notwithstanding all their differences, is under the Podophthalma.
But not a single characteristic of this section is retained by the
brood of some Prawns belonging to the genus Penëus or
in its vicinity. These quit the egg with an unsegmented ovate body,
a median frontal eye, and three pairs of natatory feet, of which
the anterior are simple, and the other two biramose—in fact,
in the larval form, so common among the lower Crustacea, to which
O. F. Müller gave the name of Nauplius. No trace of a
carapace! no trace of the paired eyes! no trace of masticating
organs near the mouth which is overarched by a helmet-like
hood!
In the case of one of these species the intermediate forms which
lead from the Nauplius to the Prawn, have been discovered in a
nearly continuous series.
The youngest Nauplius (Fig. 28) is immediately followed by forms
in which a fold of skin runs across the back behind the third pair
of feet, and four pairs of stout processes (rudiments of new limbs)
sprout forth on the ventral surface. Within the third pair of feet,
powerful mandibles are developed.
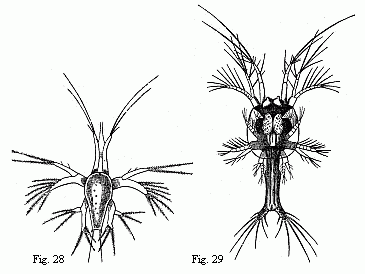
Fig. 28. Nauplius of a Prawn, magnified.
Fig. 29. Young Zoëa of the same Prawn, magnified.
In a subsequent moult the new limbs (maxillæ, and anterior
and intermediate maxillipedes) come into action, and in this way
the Nauplius becomes a Zoëa (Fig. 29), agreeing perfectly with
the Zoëa of the Crabs in the number of the appendages of the
body, although very different in form and mode of locomotion and
even in many particulars of internal structure. The chief organs of
motion are still the two anterior pairs of feet, which are slender
and furnished with long setæ; the third pair of feet loses
its branches, and becomes converted into mandibles destitute of
palpi. The labrum acquires a spine directed forward and of
considerable size, which occurs in all the Zoëæ of
allied species. The biramose maxillipedes appear to assist but
slightly in locomotion. The forked tail reminds us rather of the
forms occurring in the lower Crustacea, especially the Copepoda,
than of the spatuliform caudal plate which characterises the
Zoëæ of Alpheus, Palæmon, Hippolyte, and
other Prawns, of the Hermit Crabs, the Tatuira and the
Porcellanæ. The heart possesses only one pair of
fissures, and has no muscles traversing its interior like
trabeculæ, whilst in other Zoëæ two pairs of
fissures and an interior apparatus of trabeculæ are always
distinctly recognisable.
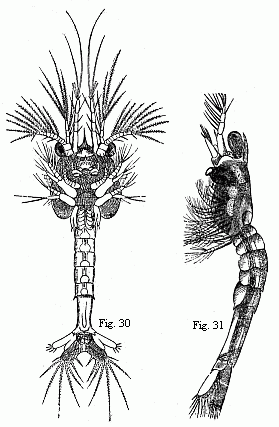
Fig. 30. Older Zoëa of the same Prawn,
magnified.
Fig. 31. Mysis-form of the same Prawn, magnified.
During this Zoëal period the paired eyes, the segments of
the middle-body and abdomen, the posterior maxillipedes, the
lateral caudal appendages and the stump-like rudiments of the feet
of the middle-body are formed (Fig. 30). The caudal appendages
sprout forth like other limbs freely on the ventral surface, whilst
in other Prawns, the Porcellanæ, etc., they are
produced in the interior of the spatuliform caudal plate.
As the feet of the middle-body come into action, simultaneously
with other profound changes, the Zoëa passes into the
Mysis- or Schizopod-form (Fig. 31). The antennæ cease to
serve for locomotion, their place is taken by the thoracic feet,
furnished with long setæ, and by the long abdomen which just
before was laboriously dragged along as a useless burden, but now,
with its powerful muscles, jerks the animal through the water in a
series of lively jumps. The anterior antennæ have lost their
long setæ, and by the side of the last (fourth) joint,
endowed with olfactory filaments, there appears a second branch,
which is at first of a single joint. The previously
multi-articulate outer branch of the posterior antennæ has
become a simple lamella, the antennal scale of the Prawn; beside
this appears the stump-like rudiment of the flagellum, probably as
a new formation, the inner branch disappearing entirely. The five
new pairs of feet are biramose, the inner branch short and simple,
the outer one longer, annulated at the end, furnished with long
setæ, and kept, as in Mysis, in constant whirling
motion. The heart acquires new fissures, and interior muscular
trabeculæ.
During the Mysis-period, the auditory organs in the basal
joint of the anterior antennæ are formed; the inner branches
of the first three pairs of feet are developed into chelæ and
the two hinder pairs into ambulatory feet; palpi sprout from the
mandibles, branchiæ on the thorax, and natatory feet on the
abdomen. The spine on the labrum becomes reduced in size. In this
way the animal gradually approaches the Prawn-form, in which the
median eye has become indistinct, the spine of the labrum, and the
outer branches of the cheliferous and ambulatory feet have been
lost, the mandibular palpi and the abdominal feet have acquired
distinct joints and setæ, and the branchiæ come into
action.
In another Prawn, the various larval states of which may be
easily recognised as belonging to the same series by the presence
of a dark-yellow, sharply-defined spot surrounding the median eye,
the youngest Zoëa (Fig. 32), probably produced from the
Nauplius, agrees in all essential particulars with the species just
described; its further development is, however, very different,
especially in that neither the feet of the middle, nor those of the
hind-body are formed simultaneously, and that a stage of
development comparable to Mysis in the number and structure of the
limbs does not occur.
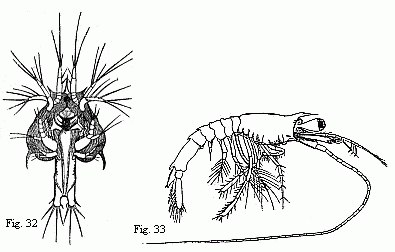
Fig. 32. Youngest (observed) Zoëa of another Prawn. The
minute buds of the third pair of maxillipedes are visible. The formation of the
abdominal segments has commenced. Paired eyes still wanting. Magnified.
Fig. 33. Older larva produced from the Zoëa represented in Fig. 32. The last
segment and the last two pairs of feet of the middle-body are wanting. Magnified.
Traces of the outer maxillipedes make their appearance betimes. Then feet
appear upon four segments of the middle-body, and these are biramose on the
three anterior segments, and simple, the inner branch being deficient, on the
fourth segment. On the inner branches the chelæ are developed; the outer
branches are lost before an inner branch has made its appearance on the fourth
segment (Fig. 32). The latter again becomes destitute of appendages, so that in
this case at an early period four, and at a later only three, segments of the
middle-body bear limbs. The fifth segment is still entirely wanting, whilst all
the abdominal segments have also acquired limbs, and this one after the other,
from before backwards. The adult animal, as shown by the three pairs of chelæ,
will certainly be very nearly allied to the preceding species.[5]
The youngest larva of the Schizopod genus Euphausia observed by
Claus, stands very near the youngest Zoëa of our Prawns; but
whilst its anterior antennæ are already biramose, and it
therefore appears to be more advanced, it still wants the middle
maxillipedes. In it also Claus found the heart furnished with only
a single pair of fissures. Do not Nauplius-like states in this case
also precede the Zoëa?
The developmental history of Mysis, the near relationship of which with
the Shrimps and Prawns has recently again been generally recognised, has been
described in detail by Van Beneden. So far as I have tested them I can only
confirm his statements. The development of the embryo commences with the
formation of the tail! This makes its appearance as a simple lobe, the dorsal
surface of which is turned towards and closely applied to that of the embryo.
(The young of other Stalk-eyed Crustacea are, as is well known, bent in the egg
in such a manner that the ventral surfaces of the anterior and posterior halves
of the body are turned towards each other,—in these, therefore, the
dorsal, and in Mysis the ventral surface appears convex.) The tail soon
acquires the furcate form with which we made acquaintance in the last
Prawn-Zoëa described. Then two pairs of thick ensiform appendages make their
appearance at the opposite end of the body, and behind these a pair of
tubercles which are easily overlooked. These are the antennæ and mandibles. The
egg-membrane now bursts, before any internal organ, or even any tissue, except
the cells of the cutaneous layer, is formed. The young animal might be called a
Nauplius; but essentially there is nothing but a rough copy of a Nauplius-skin,
almost like a new egg-membrane, within which the Mysis is developed.
The ten pairs of appendages of the fore- (maxillæ, maxillipedes) and
middle-body make their appearance simultaneously, as do the five pairs of
abdominal feet at a later period. Soon after the young Mysis casts the
Nauplius-envelope it quits the brood-pouch of the mother.[6]
For some time, owing to an undue importance being ascribed to
the want of a particular branchial cavity, Mysis, Leucifer,
and Phyllosoma were referred to the Stomapoda, which are now
again limited, as originally by Latreille, to the Mantis-shrimps
(Squilla), the Glass-shrimps (Erichthus) and their
nearest allies. Of the developmental history of these we have
hitherto been acquainted with only isolated fragments. The tracing
of the development in the egg is rendered difficult by the
circumstance, that the Mantis-shrimps do not, like the Decapoda,
carry their spawn about with them, but deposit it in the
subterranean passages inhabited by them in the form of thin, round,
yellow plates. The spawn is consequently exceedingly difficult to
procure, and unfortunately it becomes spoilt in a day when it is
removed from its natural hatching place, whilst on the contrary the
progress of development may be followed for weeks together in the
eggs of a single Crab kept in confinement. The eggs of
Squilla, like those removed from the body of the Crab, die
because they are deprived of the rapid stream of fresh water which
the mother drives through her hole for the purpose of her own
respiration.
The accompanying representation of the embryo of Squilla
shows that it possesses a long, segmented abdomen without
appendages, a bilobate tail, six pairs of limbs, and a short heart;
the latter only pulsates weakly and slowly. If it acquires more
limbs before exclusion, the youngest larva must stand on the same
level as the youngest larva of Euphausia observed by
Claus.
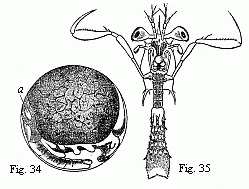
Fig. 34. Embryo of a Squilla, magnified. a.
heart.
Fig. 35. Older larva (Zoëa) of a Stomapod, magnified.
Of the two larval forms at present known which are with certainty to be
ascribed, if not to Squilla, at least to a Stomapod, I pass over the
younger one[7] as
its limbs cannot be positively interpreted, and will only mention that in it
the last three abdominal segments are still destitute of appendages. The older
larva (Fig. 35), which resembles the mature Squilla especially in the structure
of the great raptorial feet and of the preceding pair, still wants the six
pairs of feet following the raptorial feet. The corresponding body-segments are
already well developed, an unpaired eye is still present, the anterior antennæ
are already biramose, whilst the flagellum is wanting in the posterior, and the
mandibles are destitute of palpi; the four anterior abdominal segments bear
biramose natatory feet, without branchiæ; the fifth abdominal segment has no
appendages, and this is also the case with the tail, which still appears as a
simple lamina, fringed on the hinder margin with numerous short teeth. It is
evident that the larva stands essentially in the grade of Zoëa.
CHAPTER VIII.
DEVELOPMENTAL HISTORY OF EDRIOPHTHALMA.
Less varied than that of the Stalk-eyed Crustacea is
the mode of development of the Isopoda and Amphipoda, which Leach
united in the section Edriophthalma, or Crustacea with sessile
eyes.
The Rock-Slaters (Ligia) may serve as an example of the
development of the Isopoda. In these, as in Mysis, the
caudal portion of the embryo is bent not downwards, but upwards; as
in Mysis also, a larval membrane is first of all formed,
within which the Slater is developed. In Mysis this first
larval skin may be compared to a Nauplius; in Ligia it
appears like a maggot quite destitute of appendages, but produced
into a long simple tail (Fig. 37). The egg-membrane is retained
longer than in Mysis; it bursts only when the limbs of the
young Slater are already partially developed in their full number.
The dorsal surface of the Slater is united to the larval skin a
little behind the head. At this point, when the union has been
dissolved a little before the change of skin, there is a foliaceous
appendage, which exists only for a short time, and disappears
before the young Slater quits the brood-pouch of the mother.

Fig. 36. Embryo of Ligia in the egg, magnified.
D. yelk; L. liver.
Fig. 37. Maggot-like larva of
Ligia, magnified. R remains of the egg-membrane. We see on the
lower surface, from before backwards:—the anterior and posterior antennæ,
the mandibles, the anterior and posterior maxillæ, maxillipedes, six ambulatory
feet, the last segment of the middle-body destitute of appendages, five
abdominal feet, and the caudal feet.
The young animal, when it begins to take care of itself,
resembles the old ones in almost all parts, except one important
difference; it possesses only six, instead of seven pairs of
ambulatory feet; and the last segment of the middle-body is but
slightly developed and destitute of appendages. It need hardly be
mentioned that the sexual peculiarities are not yet developed, and
that in the males the hand-like enlargements of the anterior
ambulatory feet and the copulatory appendages are still
deficient.
To the question, how far the development of Ligia is repeated in the
other Isopoda, I can only give an unsatisfactory answer. The curvature of the
embryo upwards instead of downwards was met with by me as well as by Rathke in
Idothea, and likewise in Cassidina, Philoscia, Tanais, and the
Bopyridæ,—indeed, I failed to find it in none of the Isopoda examined for
this purpose. In Cassidina also the first larval skin without appendages
is easily detected; it is destitute of the long tail, but is strongly bent in
the egg, as in Ligia, and consequently cannot be mistaken for an
“inner egg-membrane.” This, however, might happen in
Philoscia, in which the larval skin is closely applied to the
egg-membrane (Fig. 38), and is only to be explained as the larval skin by a
reference to Ligia and Cassidina. The foliaceous appendage on the
back has long been known in the young of the common Water Slater
(Asellus).[1] That the last pair of feet of the thorax is
wanting in the young of the Wood-lice (Porcellionides, M.-Edw.) and
Fish-lice (Cymothoadiens, M.-Edw.) has already been noticed by
Milne-Edwards. This applies also to the Box-Slaters (Idothea), to the
viviparous Globe-Slaters (Sphæroma) and Shield-Slaters
(Cassidina), to the Bopyridæ (Bopyrus, Entoniscus, Cryptoniscus,
n.g.), and to the Cheliferous Slaters (Tanais), and therefore probably
to the great majority of the Isopoda. All the other limbs are usually well
developed in the young Isopoda. In Tanais alone, all the abdominal feet
are wanting (but not those of the tail); they are developed simultaneously with
the last pair of feet of the thorax.

Fig. 38. Embryo of a Philoscia in the egg, magnified.
Fig. 39. Embryo of Cryptoniscus planarioides, magnified.
Fig. 40. Last foot of the middle-body of the larva of Entoniscus
Porcellanæ, magnified.
The last pair of feet on the middle-body of the larva,
consequently the penultimate pair in the adult animal, is almost
always similar in structure to the preceding pair. A remarkable
exception is, however, presented in this respect by
Cryptoniscus and Entoniscus,—remarkable as a
confirmation of Darwin’s proposition that “parts
developed in an unusual manner are very variable,” for in the
peculiarly-formed pair of feet there exists the greatest possible
difference between the three species hitherto observed. In
Cryptoniscus (Fig. 39) this last foot is thin and rod-like; in
Entoniscus Cancrorum remarkably long and furnished with a
strongly thickened hand and a peculiarly constructed chela; in
Entoniscus Porcellanæ very short, imperfectly jointed,
and with a large ovate terminal joint (Fig. 40).
Some Isopods undergo a considerable change immediately before
the attainment of sexual maturity. This is the case with the males
of Tanais which have already been noticed, and, according to
Hesse, with the Pranizæ, in which both sexes are said
to pass into the form known as Anceus. But Spence Bate, a
careful observer, states that he has seen females of the form of
Praniza laden with eggs far advanced in their
development.
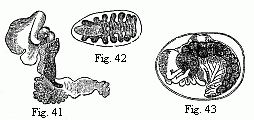
Fig. 41. Entoniscus Cancrorum, female, magnified.
Fig. 42. Cryptoniscus planarioides, female, magnified.
Fig. 43. Embryo of a Corophium, magnified.
In this order we meet for the first time with an extensive
retrograde metamorphosis as a consequence of a parasitic mode of
life. Even in some Fish-lice (Cymothoa) the young are lively
swimmers, and the adults stiff, stupid, heavy fellows, whose short
clinging feet are capable of but little movement. In the
Bopyridæ (Bopyrus, Phryxus, Kepone, etc., which might
have been conveniently left in a single genus), which are parasitic
on Crabs, Lobsters, etc., taking up their abode chiefly in the
branchial cavity, the adult females are usually quite destitute of
eyes; the antennæ are rudimentary; the broad body is
frequently unsymmetrically developed in consequence of the confined
space; its segments are more or less amalgamated with each other;
the feet are stunted, and the appendages of the abdomen transformed
from natatory feet with long setæ into foliaceous or
tongue-shaped and sometimes ramified branchiæ. In the
dwarfish males the eyes, antennæ, and feet, are usually
better preserved than in the females; but on the other hand all the
appendages of the abdomen have not unfrequently disappeared, and
sometimes every trace of segmentation. In the females of
Entoniscus, which are found in the body-cavity of Crabs and
Porcellanæ, the eyes, antennæ, and buccal organs,
the segmentation of the vermiform body, and in one species (Fig.
41) the whole of the limbs, disappear almost without leaving a
trace; and Cryptoniscus planarioides would almost be
regarded as a Flatworm rather than an Isopod, if its eggs and young
did not betray its Crustacean nature. Among the males of these
various Bopyridæ, that of Entoniscus Porcellanæ
occupies the lowest place; it is confined all its life to six pairs
of feet, which are reduced to shapeless rounded lumps.
The Amphipoda are distinguishable from the Isopoda at an early period in the
egg by the different position of the embryo, the hinder extremity of which is
bent downwards. In all the animals of this order which have been examined for
it,[2] a
peculiar structure makes its appearance very early on the anterior part of the
back, by which the embryo is attached to the “inner egg-membrane,”
and which has been called the “micropylar apparatus,” but
improperly as it seems to me.[3] It will remind us of the union of the young
Isopoda with the larval membrane and of the unpaired “adherent
organ” on the nape of the Cladocera, which is remarkably developed in
Evadne and persists throughout life; but in Daphnia pulex,
according to Leydig, although present in the young animals, disappears without
leaving a trace in the adults.
The young animal, whilst still in the egg, acquires the full number of its
segments and limbs. In cases where segments are amalgamated together, such as
the last two segments of the thorax in Dulichia, the last abdominal
segments and the tail in Gammarus ambulans and Corophium
dentatum, n. sp., and the last abdominal segments and the tail in
Brachyscelus,[4] or where one or more segments are deficient,
as in Dulichia and the Caprellæ, we find the same fusion and the
same deficiencies in young animals taken out of the brood-pouch of their
mother. Even peculiarities in the structure of the limbs, so far as they are
common to both sexes, are usually well-marked in the newly hatched young, so
that the latter generally differ from their parents only by their stouter form,
the smaller number of the antennal joints and olfactory filaments, and also of
the setæ and teeth with which the body or feet are armed, and perhaps by the
comparatively larger size of the secondary flagellum. An exception to this rule
is presented by the Hyperinæ which usually live upon Acalephæ. In these the
young and adults often have a remarkably different appearance; but even in
these there is no new formation of body-segments and limbs, but only a gradual
transformation of these parts.[5]
Figs. 44–46. Feet of a half-grown Hyperia Martinezii,
n. sp. (Named after my valued friend the amiable Spanish zoologist, M.
Francisco de Paula Martinez y Saes, at present on a voyage round the
world.)
Figs. 47–49. Feet of a nearly adult male of the same species; 44 and 47
from the first pair of anterior feet (gnathopoda); 44 and 48 from the first,
and 46 and 49 from the last pair of thoracic feet. Magnified.
Thus, in order to give a few examples, the powerful chelæ
of the antepenultimate pair of feet, of Phromina sedentaria,
are produced, according to Pagenstecher, from simple feet of
ordinary structure; and vice versà, the chelæ
on the penultimate pair of feet of the young Brachyscelus,
become converted into simple feet. In the young of the
last-mentioned genus the long head is drawn out into a conical
point and bears remarkably small eyes; in course of growth, the
latter, as in most of the Hyperinæ, attain an enormous size,
and almost entirely occupy the head, which then appears spherical,
etc.
The difference of the sexes which, in the Gammarinæ is usually expressed
chiefly in the structure of the anterior feet (gnathopoda, Sp. Bate) and in the
Hyperinæ in the structure of the antennæ, is often so great that males and
females have been described as distinct species, and even repeatedly placed in
different genera (Orchestia and Talitrus, Cerapus and
Dercothoë, Lestrigonus and Hyperia) or even families
(Hypérines anormales and Hypérines ordinaires). Nevertheless it
is only developed when the animals are nearly full-grown. Up to this period the
young resemble the females in a general way, even in some cases in which these
differ more widely than the males from the “Type” of the order.
Thus in the male Shore-hoppers (Orchestia) the second pair of the
anterior feet is provided with a powerful hand, as in the majority of the
Amphipoda, but very differently constructed in the females. The young,
nevertheless, resemble the female. Thus also,—and this is an extremely
rare case,[6]—the females of Brachyscelus are
destitute of the posterior (or inferior) antennæ; the male possesses them like
other Amphipodæ; in the young I, like Spence Bate, can find no trace of them.
It is, however, to be particularly remarked, that the
development of the sexual peculiarities does not stand still on the
attainment of sexual maturity.
For example, the younger sexually mature males of Orchestia
Tucurauna, n. sp., have slender inferior antennæ, with
the joints of the flagellum not fused together, the clasping margin
(“palm,” Sp. Bate) of the hand in the second pair of
feet is uniformly convex, the last pair of feet is slender and
similar to the preceding. Subsequently the antennæ become
thickened, two, three, or four of the first joints of the flagellum
are fused together, the palm of the hand acquires a deep
emargination near its inferior angle, and the intermediate joints
of the last pair of feet become swelled into a considerable
incrassation. No museum-zoologist would hesitate about fabricating
two distinct species, if the oldest and youngest sexually mature
males were sent to him without the uniting intermediate forms. In
the younger males of Orchestia Tucuratinga, although the
microscopic examination of their testes showed that they were
already sexually mature, the emargination of the clasping margin of
the hand (represented in Fig. 50) and the corresponding process of
the finger, are still entirely wanting. The same may be observed in
Cerapus and Caprella, and probably in all cases where
hereditary sexual differences occur.
Fig. 50. Foot of the second pair (“second pair of
gnathopoda”) of the male of Orchestia Tucurauna, magnified.
Fig. 51. Foot of the second pair (“second pair of gnathopoda”) of
the female of Orchestia Tucurauna, magnified.
Fig. 52. Male of a Bodotria, magnified. Note the long inferior antennæ,
which are closely applied to the body, and of which the apex is visible beneath
the caudal appendages.
Next to the extensive sections of the Stalk-eyed and Sessile-eyed Crustacea,
but more nearly allied to the former than to the latter, comes the remarkable
family of the Diastylidæ or Cumacea. The young, which Kröyer
took out of the brood-pouch of the female, and which attained one-fourth of the
length of their mother, resembled the adult animals almost in all parts.
Whether, as in Mysis and Ligia, a transformation occurs within
the brood-pouch, which is constructed in the same way as in Mysis, is
not known.[7]
The caudal portion of the embryo in the Diastylidæ, as I have recently
observed, is curved upwards as in the Isopoda, and the last pair of feet of the
thorax is wanting.
Equally scanty is our knowledge of the developmental history of
the Ostracoda. We know scarcely anything except that their anterior
limbs are developed before the posterior one (Zenker). The
development of Cypris has recently been observed by
Claus:—“The youngest stages are shell-bearing
Nauplius-forms.”
CHAPTER IX.
DEVELOPMENTAL HISTORY OF ENTOMOSTRACA, CIRRIPEDES, AND RHIZOCEPHALA.
The section of the Branchiopoda includes two groups differing even in their
development,—the Phyllopoda and the Cladocera. The latter minute animals,
provided with six pairs of foliaceous feet, which chiefly belong to the fresh
waters, and are diffused under similar forms over the whole world, quit the egg
with their full number of limbs. The Phyllopoda, on the contrary, in which the
number of feet varies between 10 and 60 pairs, and some of which certainly live
in the saturated lie of salterns and natron-lakes, but of which only one rather
divergent genus (Nebalia) is found in the sea,[1] have to undergo a
metamorphosis. Mecznikow has recently observed the development of
Nebalia, and concludes from his observations “that Nebalia,
during its embryonal life, passes through the Nauplius- and Zoëa-stages, which
in the Decapoda occur partly (in Penëus) in the free state.”
“Therefore,” says he, “I regard Nebalia as a Phyllopodiform
Decapod.” The youngest larvæ [of the Phyllopoda] are Nauplii, which we
have already met with exceptionally in some Prawns, and which we shall now find
reproduced almost without exception. The body-segments and feet, which are
sometimes so numerous, are formed gradually from before backwards, without the
indication of any sharply-discriminated regions of the body either by the time
of their appearance or by their form. All the feet are essentially constructed
in the same manner and resemble the maxillæ of the higher Crustacea.[2] We might
regard the Phyllopoda as Zoëæ which have not arrived at the formation of a
peculiarly endowed abdomen or thorax, and instead of these have repeatedly
reproduced the appendages which first follow the Nauplius-limbs.
Of the Copepoda—some of which, living in a free state, people the fresh
waters, and in far more multifarious forms the sea, whilst others, as
parasites, infest animals of the most various classes and often become
wonderfully deformed—the developmental history, like their entire natural
history, was, until lately, in a very unsatisfactory state. It is true, that we
long ago knew that the Cyclopes of our fresh waters were excluded in the
Nauplius-form, and that we were acquainted with some others of their young
states; we had learnt, through Nordmann, that the same earliest form belonged
to several parasitic Crustacea, which had previously passed, almost
universally, as worms; but the connecting intermediate forms which would have
permitted us to refer the regions of the body and the limbs of the larvæ to
those of the adult animal, were wanting. The comprehensive and careful
investigations of Claus have filled up this deficiency in our knowledge, and
rendered the section of the Copepoda one of the best known in the whole class.
The following statements are derived from the works of this able naturalist.
From the abundance of valuable materials which they contain I select only those
which are indispensable for the comprehension of the development of the
Crustacea in general, because, in what relates to the Copepoda in particular,
the facts have already been placed in the proper light by the representation of
their most recent investigator, and must appear to any one whose eyes are open,
as important evidence in favour of the Darwinian theory.[3]
All the larvæ of the free Copepoda investigated by Claus, have, at the earliest
period, three pairs of limbs (the future antennæ and mandibles), the anterior
with a single, and the two following ones with a double series of joints, or
branchiæ. The unpaired eye, labrum, and mouth, already occupy their permanent
positions. The posterior portion, which is usually short and destitute of
limbs, bears two terminal setæ, between which the anus is situated. The form in
this Nauplius-brood is extremely various,—it is sometimes compressed
laterally, sometimes flat,—sometimes elongated, sometimes oval, sometimes
round or even broader than long, and so forth. The changes which the first
larval stages undergo during the progress of growth, consist essentially in an
extension of the body and the sprouting forth of new limbs. “The
following stage already displays a fourth pair of extremities, the future
maxillæ.” Then follow at once three new pairs of limbs (the maxillipedes
and the two anterior pairs of natatory feet). The larva still continues like a
Nauplius, as the three anterior pairs of limbs represent rowing feet; at the
next moult it is converted into the youngest Cyclops-like state, when it
resembles the adult animal in the structure of the antennæ and buccal organs,
although the number of limbs and body segments is still much less, for only the
rudiments of the third and fourth pairs of natatory feet have made their
appearance in the form of cushions fringed with setæ, and the body consists of
the oval cephalothorax, the second, third, and fourth thoracic segments, and an
elongated terminal joint. In the Cyclopidæ the posterior antennæ have lost
their secondary branch, and the mandibles have completely thrown off the
previously existing natatory feet, whilst in the other families these
appendages persist, more or less altered. “Beyond this stage of free
development, many forms of the parasitic Copepoda, such as Lernanthropus
and Chondracanthus, do not pass, as they do not acquire the third and
fourth pairs of limbs, nor does a separation of the fifth thoracic segment from
the abdomen take place; others (Achtheres) even fall to a lower grade by
the subsequent loss of the two pairs of natatory feet. But all free Copepoda,
and most of the parasitic Crustacea, pass through a longer or shorter series of
stages of development, in which the limbs acquire a higher degree of division
into joints in continuous sequence, the posterior pairs of feet are developed,
and the last thoracic segment and the different abdominal segments are
successively separated from the common terminal portion.”
Fig. 53 and 54. Nauplii of Copepoda, the former magnified,
the latter magnified 2x.
Fig. 55. Nauplius of Tetraclita porosa after the first moult, magnified.
The brain is seen surrounding the eye, and from it the olfactory filaments
issue; behind it are some delicate muscles passing to the buccal hood.
There is only one thing more to be indicated in the developmental history of
the parasitic Crustacea, namely that some of them, such as Achtheres
percarum, certainly quit the egg like the rest in a Nauplius-like form,
inasmuch as the plump, oval, astomatous body bears two pairs of simple rowing
feet, and behind these, as traces of the third pair, two inflations furnished
each with a long seta, but that beneath this Nauplius-skin a very different
larva lies ready prepared, which in a few hours bursts its clumsy envelope and
then makes its appearance in a form “which agrees in the segmentation of
the body and in the development of the extremities with the first
Cyclops-stage” (Claus). The entire series of Nauplius-stages which
are passed through by the free Copepoda, are in this case completely
over-leapt.
A final and very peculiar section of the Crustacea is formed by the two orders
of the Cirripedia and Rhizocephala.[4]
In these also the brood bursts out in the Nauplius-form, and speedily strips
off its earliest larva-skin which is distinguished by no peculiarities worth
noticing. Here also we find again the same pyriform shape of the unsegmented
body, the same number and structure of the feet, the same position of the
median eye (which, however, is wanting in Sacculina purpurea, and
according to Darwin in some species of Lepas), and the same position of
the “buccal hood,” as in the Nauplii of the Prawns and Copepoda.
From the latter the Nauplii of the Cirripedia and Rhizocephala are
distinguished by the possession of a dorsal shield or carapace, which sometimes
(Sacculina purpurea) projects far beyond the body all round; and they
are distinguished not only from other Nauplii, but as far as I know from all
other Crustacea, by the circumstance that structures which are elsewhere
combined with the two anterior limbs (antennæ), here occur separated from them.
The anterior antennæ of the Copepoda, Cladocera, Phyllopoda (Leydig, Claus),
Ostracoda (at least the Cypridinæ), Diastylidæ, Edriophthalma, and
Podophthalma, with few exceptions relating to terrestrial animals or parasites,
bear peculiar filaments which I have already repeatedly mentioned as
“olfactory filaments.” A pair of similar filaments spring, in the
larvæ of the Cirripedia and Rhizocephala, directly from the brain.
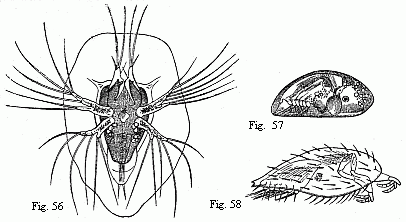
Fig. 56. Nauplius of Sacculina purpurea, shortly
before the second moult, magnified. We may recognise in the first pair of feet
the future adherent feet, and in the abdomen six pairs of natatory feet with
long setæ.
Fig. 57. Pupa of a Balanide (Chthamalus ?), magnified. The adherent feet
are retracted within the rather opaque anterior part of the shell.
Fig. 58. Pupa of Sacculina purpurea, magnified. The filaments on the
adherent feet may be the commencements of the future roots.
At the base of the inferior antennæ in the Decapoda the so-called
“green-gland” has its opening; in the Macrura at the end of a
conical process. A similar conical process with an efferent duct traversing it
is very striking in most of the Amphipoda. In the Ostracoda, Zenker describes a
gland situated in the base of the inferior antennæ, and opening at the
extremity of an extraordinarily long “spine.” In the Nauplii of
Cyclops and Cyclopsine, Claus finds pale
“shell-glands,” which commence in the intermediate pair of limbs
(the posterior antennæ). On the other hand in the Nauplii of the Cirripedia and
Rhizocephala the “shell-glands” open at the ends of conical
processes, sometimes of most remarkable length, which spring from the angles of
the broad frontal margin, and have been interpreted sometimes as antennæ
(Burmeister, Darwin) and sometimes as mere “horns of the carapace”
(Krohn). The connexion of the “shell-glands” with the frontal horns
has been recognised unmistakably in the larvæ of Lepas, and indeed the
resemblance of the frontal horns with the conical processes on the inferior
antennæ of the Amphipoda, is complete throughout.[5]
Notwithstanding their agreement in this important peculiarity, the Nauplii of
these two orders present material differences in many other particulars. The
abdomen of the young Cirripede is produced beneath the anus into a long
tail-like appendage which is furcate at the extremity, and over the anus there
is a second long, spine-like process; the abdomen in the Rhizocephala
terminates in two short points,—in a “moveable caudal fork, as in
the Rotatoria,” (O. Schmidt). The young Cirripedes have a mouth, stomach,
intestine, and anus, and their two posterior pairs of limbs are beset with
multifarious teeth, setæ, and hooks, which certainly assist in the inception of
nourishment. All this is wanting in the young Rhizocephala. The Nauplii of the
Cirripedia have to undergo several moults whilst in that form; the Nauplii of
the Rhizocephala, being astomatous, cannot of course live long as Nauplii, and
in the course of only a few days they become transformed into equally
astomatous “pupæ,” as Darwin calls them.
The carapace folds itself together, so that the little animal acquires the
aspect of a bivalve shell, the foremost limbs become transformed into very
peculiar adherent feet (“prehensile antennæ,” Darwin), and the two
following pairs are cast off; like the frontal horns. On the abdomen six pairs
of powerful biramose natatory feet with long setæ have been formed beneath the
Nauplius-skin, and behind these are two short, setigerous caudal appendages
(Fig. 58).
The pupæ of the Cirripedia (Fig. 57), which are likewise astomatous, agree
completely in all these parts with those of the Rhizocephala, even to the
minutest details of the segmentation and bristling of the natatory feet;[6] they are
especially distinguished from them by the possession of a pair of composite
eyes. Sometimes also traces of the frontal horns seem to persist.[7]
As the Cirripedia and Rhizocephala now in general resemble each other far more
than in their Nauplius-state, this is also the case with the individual members
of each of the two orders.
The pupæ in both orders attach themselves by means of the adherent feet; those
of the Cirripedes to rocks, shells, turtles, drift-wood, ships,
etc.,—those of the Rhizocephala to the abdomen of Crabs,
Porcellanæ, and Hermit Crabs. The carapace of the Cirripedes becomes
converted, as is well-known, into a peculiar test, on account of which they
were formerly placed among the Mollusca, and the natatory feet grow into long
cirri, which whirl nourishment towards the mouth, which is now open. The
Rhizocephala remain astomatous; they lose all their limbs completely, and
appear as sausage-like, sack-shaped or discoidal excrescences of their host,
filled with ova (Figs. 59, 60); from the point of attachment closed tubes,
ramified like roots, sink into the interior of the host, twisting round its
intestine, or becoming diffused among the sac-like tubes of its liver. The only
manifestations of life which persist in these non plus ultras in the
series of retrogressively metamorphosed Crustacea, are powerful contractions of
the roots, and an alternate expansion and contraction of the body, in
consequence of which water flows into the brood-cavity and is again expelled,
through a wide orifice.[8]
Fig. 59. Young of Peltogaster socialis on the abdomen
of a small Hermit Crab; in one of them the fasciculately ramified roots in the
liver of the Crab are shown. Animal and roots deep yellow.
Fig. 60. Young Sacculina purpurea with its roots; the animal purple-red,
the roots dark grass-green. Magnified.
Figs. 61–63. Eggs of Tetraclita porosa in segmentation, magnified.
The larger of the two first-formed spheres of segmentation is always turned
towards the pointed end of the egg.
Fig. 64. Egg of Lernæodiscus Porcellanæ, in segmentation, magnified.
Out of several Cirripedes, which are anomalous both in structure and
development, Cryptophialus minutus must be mentioned here; Darwin found
it in great quantities together in the shell of Concholepas peruviana on
the Chonos Islands. The egg, which is at first elliptical, soon, according to
Darwin, becomes broader at the anterior extremity, and acquires three
club-shaped horns, one at each anterior angle and one behind; no internal parts
can as yet be detected. Subsequently the posterior horn disappears, and the
adherent feet may be recognised within the anterior ones. From this
“egg-like larva”—(Darwin says of it, “I hardly know
what to call it”)—the pupa is directly produced. Its carapace is
but slightly compressed laterally and hairy, as in Sacculina purpurea;
the adherent feet are of considerable size, and the natatory feet are wanting,
as, in the adult animal, are the corresponding cirri. As I learn from Mr.
Spence Bate, the Nauplius-stage appears to be overleaped and the larvæ to leave
the egg in the pupa-form, in the case of a Rhizocephalon (Peltogaster ?)
found by Dr. Powell in the Mauritius.
I will conclude this general view with a few words upon the earliest processes
in the development of the Crustacea. Until recently it was regarded as a
general rule that, by the partial segmentation of the vitellus a germinal disc
was formed, and in this, corresponding to the ventral surface of the embryo, a
primitive band. We now know that in the Copepoda (Claus), in the Rhizocephala
(Fig. 64), and, as I can add, in the Cirripedia (Figs. 61–63) the
segmentation is complete, and the embryos are sketched out in their complete
form without any preceding primitive band. Probably the latter will always be
the case where the young are hatched as true Nauplii (and not merely
with a Nauplius-skin, as in Achtheres). The two modes of development may
occur in very closely allied animals, as is proved by Achtheres among
the Copepoda.[9]
CHAPTER X.
ON THE PRINCIPLES OF CLASSIFICATION.
Perhaps some one else, more fortunate than myself, may be able, even without
Darwin, to find the guiding clue through the confusion of developmental forms,
now so totally different in the nearest allies, now so surprisingly similar in
members of the most distant groups, which we have just cursorily reviewed.
Perhaps a sharper eye may be able, with Agassiz, to make out “the plan
established from the beginning by the Creator,”[1] who may have written here,
as a Portuguese proverb says “straight in crooked lines.”[2] I cannot
but think that we can scarcely speak of a general plan, or typical mode of
development of the Crustacea, differentiated according to the separate
Sections, Orders, and Families, when, for example, among the Macrura, the River
Crayfish leaves the egg in its permanent form; the Lobster with Schizopodal
feet; Palæmon, like the Crabs, as a Zoëa; and Penéus, like the
Cirripedes, as a Nauplius,—and when, still, within this same sub-order
Macrura, Palinurus, Mysis and Euphausia again present different
young forms,—when new limbs sometimes sprout forth as free rudiments on
the ventral surface, and are sometimes formed beneath the skin which passes
smoothly over them, and both modes of development are found in different limbs
of the same animal and in the same pair of limbs in different
animals,—when in the Podophthalma the limbs of the thorax and abdomen
make their appearance sometimes simultaneously, or sometimes the former and
sometimes the latter first, and when further in each of the two groups the
pairs sometimes all appear together, and sometimes one after the
other,—when, among the Hyperina, a simple foot becomes a chela in
Phronima and a chela a simple foot in Brachyscelus, etc.
And yet, according to the teaching of the school, it is precisely in youth,
precisely in the course of development, that the “Type” is mostly
openly displayed. But let us hear what the Old School has to tell us as to the
significance of developmental history, and its relation to comparative anatomy
and systematic zoology.
Let two of its most approved masters speak.
“Whilst comparative anatomy,” said Johannes Müller, in 1844, in his
lectures upon this science (and the opinions of my memorable teacher were for
many years my own), “whilst comparative anatomy shows us the infinitely
multifarious formation of the same organ in the Animal Kingdom, it furnishes us
at the same time with the means, by the comparison of these various forms, of
recognising the truly essential, the type of these organs, and separating
therefrom everything unessential. In this, developmental history serves it as a
check or test. Thus, as the idea of development is not that of mere increase of
size, but that of progress from what is not yet distinguished, but which
potentially contains the distinction in itself, to the actually
distinct,—it is clear, that the less an organ is developed, so much the
more does it approach the type, and that, during its development, it more and
more acquires peculiarities. The types discovered by comparative anatomy and
developmental history must therefore agree.”
Then, after Johannes Müller has combated the idea of a graduated scale of
animals, and of the passage through several animal grades during development,
he continues:—“What is true in this idea is, that every embryo at
first bears only the type of its section, from which the type of the Class,
Order, etc., is only afterwards developed.”
In 1856, in an elementary work,[3] in which it is usual to admit only what are
regarded as the assured acquisitions of science, Agassiz expresses himself as
follows:—
“The ovarian eggs of all animals are perfectly identical, small
cells with a vitellus, germinal vesicle and germinal spot” (§ 278).
“The organs of the body are formed in the sequence of their organic
importance; the most essential always appear first. Thus the organs of
vegetative life, the intestine, etc., appear later than those of animal life,
the nervous system, skeleton, etc., and these in turn are preceded by the more
general phenomena belonging to the animal as such” (§ 318).
“Thus, in Fishes, the first changes consist in the segmentation of the
vitellus and the formation of a germ, processes which are common to all classes
of animals. Then the dorsal furrow, characteristic of the Vertebrate,
appears—the brain, the organs of the senses; at a later period are formed
the intestine, the limbs, and the permanent form of the respiratory organs,
from which the class is recognised with certainty. It is only after exclusion
that the peculiarities of the structure of the teeth and fins indicate the
genus and species” (§ 319). “Hence the embryos of different
animals resemble each other the more, the younger they are” (§
320). “Consequently the high importance of developmental history is
indubitable. For, if the formation of the organs takes place in the order
corresponding to their importance, this sequence must of itself be a criterion
of their comparative value in classification. The peculiarities which
appear earlier should be considered of higher value than those which appear
subsequently” (§ 321). “A system, in order to be true and
natural, must agree with the sequence of the organs in the development of the
embryo” (§ 322).
I do not know whether any one at the present day will be inclined to subscribe
to this proposition in its whole extent.[4] It is certain, however, that views
essentially similar are still to be met with everywhere in discussions on
classification, and that even within the last few years, the very sparingly
successful attempts to employ developmental history as the foundation of
classification have been repeated.
But how do these propositions agree with our observations on the developmental
history of the Crustacea? That these observations relate for the most part to
their “free metamorphosis” after their quitting the egg, cannot
prejudice their application to the propositions enunciated especially with
regard to “embryonal development” in the egg; for Agassiz himself
points out (§ 391) that both kinds of change are of the same nature and of
equal importance and that no “radical distinction” is produced by
the circumstance that the former take place before and the latter after birth.
“The ovarian eggs of all animals are identical, small cells with
vitellus, germinal vesicle and germinal spot.” Yes, somewhat as all
Insects are identical, small animals with head, thorax, and abdomen; that is to
say if, only noticing what is common to them, we leave out of consideration the
difference of their development, the presence or absence and the multifarious
structure of the vitelline membrane, the varying composition of the vitellus,
the different number and formation of the germinal spots, etc. Numerous
examples, which might easily be augmented, of such profound differences, are
furnished by Leydig’s ‘Lehrbuch der Histologie.’ In the
Crustacea the ovarian eggs actually sometimes furnish excellent characters for
the discrimination of species of the same genus; thus, for example, in one
Porcellana of this country they are blackish-green, in a second deep
blood-red, and in a third dark yellow; and within the limits of the same order
they present considerable differences in size, which, as Van Beneden and Claus
have already pointed out, stands in intimate connexion with the subsequent mode
of development.
“The organs of the body are formed in the sequence of their organic
importance; the most essential always appear first.” This proposition
might be characterised à priori as undemonstrable, since it is
impossible either in general, or for any particular animal, to establish a
sequence of importance amongst equally indispensable parts. Which is the more
important, the lung or the heart—the liver or the kidney?—the
artery or the vein? Instead of giving the preference, with Agassiz, to the
organs of animal life, we might with equal justice give it to those of
vegetative life, as the latter are conceivable without the former, but not the
former without the latter. We might urge that, according to this proposition,
provisional organs as the first produced must exceed the later-formed permanent
organs in importance.
But let us stick to the Crustacea. In Polyphemus Leydig finds the first
traces of the intestinal tube even during segmentation. In Mysis a
provisional tail is first formed, and in Ligia a maggot-like larva-skin.
The simple median eye appears earlier, and would therefore be more important
than the compound paired eyes; the scale of the antennæ in the Prawns would be
more important than the flagellum; the maxillipedes of the Decapoda would be
more important than the chelæ and ambulatory feet, and the anterior six pairs
of feet in the Isopoda, than the precisely similarly formed seventh pair; in
the Amphipoda the most important of all organs would be the “micropylar
apparatus,” which disappears without leaving a trace soon after hatching;
in Cyclops the setæ of the tail would be more important than all the
natatory feet; in the Cirripedia the posterior antennæ, as to which we do not
know what becomes of them, would be more important than the cirri, and so
forth. The most unimportant of all organs would be the sexual organs, and the
most essential peculiarity would consist in colour, which is to be referred
back to the ovarian egg.
“The embryos, or young states of different animals, resemble each
other the more, the younger they are,” or, as Johannes Müller
expresses it, “they approach the more closely to the common
type.” Different as may be the ideas connected with the word
“type,” no one will dispute that the typical form of the
penultimate pair of feet in the Amphipoda is that of a simple ambulatory foot,
and not that of a chela, for the latter occurs in no single adult Amphipod; we
know it only in the young of the genus Brachyscelus, which therefore in
this respect undoubtedly depart more widely than the adults from the type of
their order. This applies also to the young males of the Shore-hoppers
(Orchestia) with regard to the second pair of anterior feet
(gnathopoda). In like manner no one will hesitate to accept the
possession of seven pairs of feet as a “typical” peculiarity of the
Edriophthalma, which Agassiz, on this account, names Tetradecapoda; the young
Isopoda, which are Dodecapoda, are also in this respect further from the
“type” than the adults.
It is certainly a rule, and this Darwin’s theory would lead us to expect,
that in the progress of development those forms which are at first similar
gradually depart further from each other; but here, as in other classes, the
exceptions, for which the Old School has no explanation, are numerous. Not
unfrequently we might indeed directly reverse the proposition and assert that
the difference becomes the greater, the further we go back in the development,
and this not only in those cases in which one of two nearly allied species is
directly developed, and the other passes through several larval stages, such as
the common Crayfish and the Prawns which are produced from Nauplius-brood. The
same may be said, for example, of the Isopoda and Amphipoda. In the adult
animals the number of limbs is the same; at the first sight of a
Cyrtophium or a Dulichia, and even after the careful examination
of a Tanais, we may be in doubt whether we have an Isopod or an Amphipod
before us; in the newly-hatched young the number of limbs is different, and if
we go back to their existence in the egg, the most passing glance to see
whether the curvature is upwards or downwards suffices to distinguish even the
youngest embryos of the two orders.
In other instances, the courses which lead from a similar starting-point to a
similar goal, separate widely in the middle of the development, as in the
Prawns with Nauplius-brood already described.
Finally, so that even the last possibility may be exhausted, it sometimes
happens that the greatest similarity occurs in the middle of the development.
The most striking example of this is furnished by the Cirripedia and
Rhizocephala, whether we compare the two orders or the members of each with one
another; from a segmentation quite different in its course (see Figs.
61–64) proceed different forms of Nauplius, these become converted into
exceedingly similar pupæ, and from the pupæ again proceed sexually mature
animals, differing from each other toto cœlo.
“If the formation of the organs occurs in the order corresponding to
their importance, this sequence must of itself be a criterion of their
comparative value in classification.” THAT IS TO SAY, SUPPOSING THE
PHYSIOLOGICAL AND CLASSIFICATIONAL VALUE OF AN ORGAN TO COINCIDE! Just as in
Christian countries there is a catechismal morality, which every one has upon
his lips, but no one considers himself bound to follow, or expects to see
followed by anybody else, so also has Zoology its dogmas, which are as
universally acknowledged, as they are disregarded in practice. Such a dogma as
this is the supposition tacitly made by Agassiz. Of a hundred who feel
themselves compelled to give their systematic confession of faith as the
introduction to a Manual or Monographic Memoir, ninety-nine will commence by
saying that a natural system cannot be founded upon a single character, but
that it has to take into account all characters, and the general structure of
the animal, but that we must not simply sum up these characters like equivalent
magnitudes, that we must not count but weigh them, and determine the importance
to be ascribed to each of them according to its physiological significance.
This is probably followed by a little jingle of words in general terms on the
comparative importance of animal and vegetative organs, circulation,
respiration, and the like. But when we come to the work itself, to the
discrimination and arrangement of the species, genera, families, etc., in all
probability not one of the ninety-nine will pay the least attention to these
fine rules, or undertake the hopeless attempt to carry them out in detail.
Agassiz, for example, like Cuvier, and in opposition to the majority of the
German and English zoologists, regards the Radiata as one of the great primary
divisions of the Animal Kingdom, although no one knows anything about the
significance of the radiate arrangement in the life of these animals, and
notwithstanding that the radiate Echinodermata are produced from bilateral
larvæ. The “true Fishes” are divided by him into Ctenoids and
Cycloids, according as the posterior margin of their scales is denticulated or
smooth, a circumstance the importance of which to the animal must be infinitely
small, in comparison to the peculiarities of the dentition, formation of the
fins, number of vertebræ, etc.
And, to return to our Class of the Crustacea, has any particular attention been
paid in their classification to the distinctions prevailing in the “most
essential organs”? For instance, to the nervous system? In the Corycæidæ,
Claus found all the ventral ganglia fused together into a single broad mass,
and in the Calanidæ a long ventral chain of ganglia,—the former,
therefore, in this respect resembling the Spider Crabs and the latter the
Lobster; but no one would dream on this account of supposing that there was a
relationship between the Corycæidæ and the Crabs, or the Calanidæ and the
Lobsters.—Or to the organs of circulation? We have among the Copepoda,
the Cyclopidæ and Corycæidæ without a heart, side by side with the Calanidæ and
Pontellidæ with a heart. And in the same way among the Ostracoda, the
Cypridinæ, which I find possess a heart, place themselves side by side
with Cypris and Cythere which have no such organ.—Or to the
respiratory apparatus? Milne-Edwards did this when he separated Mysis
and Leucifer from the Decapoda, but he himself afterwards saw that this
was an error. In one Cypridina I find branchiæ of considerable size,
which are entirely wanting in another species, but this does not appear to me
to be a reason for separating these species even generically.
On the other hand, what do we know of the physiological significance of the
number of segments, and all the other matters which we are accustomed to regard
as typical peculiarities of the different organs, and to which we usually
ascribe the highest systematic value?
“Those peculiarities which first appear, should be more highly
estimated than those which appear subsequently. A system, in order to be true
and natural, must agree with the sequence of the organs in the development of
the embryo.” If the earlier manifested peculiarities are to be
estimated more highly than those which afterwards make their appearance, then
in those cases in which the structure of the adult animal requires one position
in the system, and that of the larva another, the latter and not the former
must decide the point. As the Lernææ and Cirripedes, on account of their
Nauplius-brood, were separated from their previous connexions and referred to
the Crustacea, we shall, for the same reason, have to separate Penëus
from the Prawns and unite it with the Copepoda and Cirripedia. But the most
zealous embryomaniac would probably shrink from this course.
A “true and natural system” of the Crustacea to be in accordance
with the sequence of the phenomena would have to take into account in the first
place the various modes of segmentation, then the position of the embryo, next
the number of limbs produced within the egg and so forth, and might be
represented somewhat as follows:—
CLASSIS CRUSTACEA.
Sub-class I. HOLOSCHISTA.—Segmentation complete. No
primitive band. Nauplius-brood.
Ord. 1. Ceratometopa.)—Nauplius with frontal horns. (Cirripedia,
Rhizocephala.)
Ord. 2. LEIOMETOPA.—Nauplius without frontal horns.
(Copepoda, without Achtheus, etc., Phyllopoda, Penëus.)
Sub-class II. HEMISCHISTA.—Segmentation not complete.
A. Nototropa.—Embryo bent upwards.
Ord. 3. Protura.—The tail is first formed. (Mysis.)
Ord. 3. Saccomorpha.—A maggot-like larva-skin is first formed.
(Isopoda.)
B. Gasterotropa.—Embryo bent ventrally.
Ord. 5. Zoëogona.—Full number of limbs not produced in the egg.
Zoëa-brood. (The majority of the Podophthalmata.)
Ord. 6. Ametabola.—Full number of limbs produced in the egg.
(Astacus, Gecarcinus, Amphipoda less Hyperia ?)
This sample may suffice. The farther we go into details in this direction, the
more brilliantly, as may easily be imagined, does the naturalness of such an
arrangement as this force itself upon us.
All things considered, we may apply the judgment which Agassiz pronounced upon
Darwin’s theory, with far greater justice to the propositions just
examined:—“No theory,” says he, “however plausible it
may be, can be admitted in science, unless it is supported by facts.”
CHAPTER XI.
ON THE PROGRESS OF EVOLUTION.
From this scarcely unavoidable but unsatisfactory side-glance upon the old
school, which looks down with so great an air of superiority upon Darwin's
“intellectual dream” and the “giddy enthusiasm” of its
friends, I turn to the more congenial task of considering the developmental
history of the Crustacea from the point of view of the Darwinian theory.
Darwin himself, in the thirteenth chapter of his book, has already discussed
the conclusions derived from his hypotheses in the domain of developmental
history. For a more detailed application of them, however, it is necessary in
the first place to trace these general conclusions a little further than he has
there done.
The changes by which young animals depart from their parents, and the gradual
accumulation of which causes the production of new species, genera, and
families, may occur at an earlier or later period of life,—in the young
state, or at the period of sexual maturity. For the latter is by no means
always, as in the Insecta, a period of repose; most other animals even then
continue to grow and to undergo changes. (See above, the remarks on the males
of the Amphipoda.) Some variations, indeed, from their very nature, can only
occur when the young animal has attained the adult stage of development. Thus
the Sea Caterpillars (Polynoë) at first possess only a few
body-segments, which, during development, gradually increase to a number which
is different in different species, but constant in the same species; now before
a young animal could exceed the number of segments of its parents, it must of
course have attained that number. We may assume a similar supplementary
progress wherever the deviation of the descendants consists in an addition of
new segments and limbs.
Descendants therefore reach a new goal, either by deviating sooner or later
whilst still on the way towards the form of their parents, or by passing along
this course without deviation, but then, instead of standing still, advance
still farther.
The former mode will have had a predominant action where the posterity of
common ancestors constitutes a group of forms standing upon the same level in
essential features, as the whole of the Amphipoda, Crabs, or Birds. On the
other hand we are led to the assumption of the second mode of progress, when we
seek to deduce from a common original form, animals some of which agree with
young states of others.
In the former case the developmental history of the descendants can only agree
with that of their ancestors up to a certain point at which their courses
separate,—as to their structure in the adult state it will teach us
nothing. In the second case the entire development of the progenitors is
also passed through by the descendants, and, therefore, so far as the
production of a species depends upon this second mode of progress, the
historical development of the species will be mirrored in its developmental
history. In the short period of a few weeks or months, the changing forms
of the embryo and larvæ will pass before us, a more or less complete and more
or less true picture of the transformations through which the species, in the
course of untold thousands of years, has struggled up to its present state.
Figs. 65–67. Young Tubicolar worms, magnified with the
simple lens: 65.[1] Without operculum, Protula-stage; 66.
With a barbate opercular peduncle, Filograna-stage; 67. With a naked
opercular peduncle, Serpula-stage.
One of the simplest examples is furnished by the development of the Tubicolar
Annelids; but from its very simplicity it appears well adapted to open the eyes
of many who, perhaps, would rather not see, and it may therefore find a place
here. Three years ago I found on the walls of one of my glasses some small
worm-tubes (Fig. 65), the inhabitants of which bore three pairs of barbate
branchial filaments, and had no operculum. According to this we should have
been obliged to refer them to the genus Protula. A few days afterwards
one of the branchial filaments had become thickened at the extremity into a
clavate operculum (Fig. 66), when the animals reminded me, by the barbate
opercular peduncle, of the genus Filograna, only that the latter
possesses two opercula. In three days more, during which a new pair of
branchial filaments had sprouted forth, the opercular peduncle had lost its
lateral filaments (Fig. 67), and the worms had become Serpulæ. Here the
supposition at once presents itself that the primitive tubicolar worm was a
Protula,—that some of its descendants, which had already become
developed into perfect Protulæ, subsequently improved themselves by the
formation of an operculum which might protect their tubes from inimical
intruders,—and that subsequent descendants of these latter finally lost
the lateral filaments of the opercular peduncle, which they, like their
ancestors, had developed.
What say the schools to this case? Whence and for what purpose, if the
Serpulæ were produced or created as ready-formed species, these lateral
filaments of the opercular peduncle? To allow them to sprout forth merely for
the sake of an invariable plan of structure, even when they must be immediately
retracted again as superfluous, would certainly be an evidence rather of
childish trifling or dictatorial pedantry, than of infinite wisdom. But no, I
am mistaken; from the beginning of all things the Creator knew, that one day
the inquisitive children of men would grope about after analogies and
homologies, and that Christian naturalists would busy themselves with thinking
out his Creative ideas; at any rate, in order to facilitate the discernment by
the former that the opercular peduncle of the Serpulæ is homologous with
a branchial filament, He allowed it to make a detour in its development, and
pass through the form of a barbate branchial filament.
The historical record preserved in developmental history is gradually
EFFACED as the development strikes into a constantly
straighter course from the egg to the perfect animal, and it is frequently
SOPHISTICATED by the struggle for existence which the
free-living larvæ have to undergo.
Thus as the law of inheritance is by no means strict, as it gives room for
individual variations with regard to the form of the parents, this is also the
case with the succession in time of the developmental processes. Every father
of a family who has taken notice of such matters, is well aware that even in
children of the same parents, the teeth, for example, are not cut or changed,
either at the same age, or in the same order. Now in general it will be useful
to an animal to obtain as early as possible those advantages by which it
sustains itself in the struggle for existence. A precocious appearance of
peculiarities originally acquired at a later period will generally be
advantageous, and their retarded appearance disadvantageous; the former, when
it appears accidentally, will be preserved by natural selection. It is the same
with every change which gives to the larval stages, rendered multifarious by
crossed and oblique characters, a more straightforward direction, simplifies
and abridges the process of development, and forces it back to an earlier
period of life, and finally into the life of the egg.
As this conversion of a development passing through different young states into
a more direct one, is not the consequence of a mysterious inherent impulse, but
dependent upon advances accidentally presenting themselves, it may take place
in the most nearly allied animals in the most various ways, and require very
different periods of time for its completion. There is one thing, however, that
must not be overlooked here. The historical development of a species can hardly
ever have taken place in a continuously uniform flow; periods of rest will have
alternated with periods of rapid progress. But forms, which in periods of rapid
progress were severed from others after a short duration, must have impressed
themselves less deeply upon the developmental history of their descendants,
than those which repeated themselves unchanged, through a long series of
successive generations in periods of rest. These more fixed forms, less
inclined to variation, will present a more tenacious resistance in the
transition to direct development, and will maintain themselves in a more
uniform manner and to the last, however different may be the course of this
process in other respects.
In general, as already stated, it will be advantageous to the young to commence
the struggle for existence in the form of their parents and furnished with all
their advantages—in general, but not without exceptions. It is perfectly
clear that a brood capable of locomotion is almost indispensable to attached
animals, and that the larvæ of sluggish Mollusca, or of worms burrowing in the
ground, etc., by swarming briskly through the sea perform essential services by
dispersing the species over wider spaces. In other cases a metamorphosis is
rendered indispensable by the circumstance that a division of labour has been
set up between the various periods of life; for example, that the larvæ have
exclusively taken upon themselves the business of nourishment. A further
circumstance to be taken into consideration is the size of the eggs,—a
simpler structure may be produced with less material than a more compound
one,—the more imperfect the larva, the smaller may the egg be, and the
larger is the number of these that the mother can furnish with the same
expenditure of material. As a rule, I believe indeed, this advantage of a more
numerous brood will not by any means outweigh that of a more perfect brood, but
it will do so in those cases in which the chief difficulty of the young animals
consists in finding a suitable place for their development, and in which,
therefore, it is of importance to disperse the greatest possible number of
germs, as in many parasites.
As the conversion of the original development with metamorphosis into direct
development is here under discussion, this may be the proper place to say a
word as to the already indicated absence of metamorphosis in fresh-water and
terrestrial animals the marine allies of which still undergo a transformation.
This circumstance seems to be explicable in two ways. Either species without a
metamorphosis migrated especially into the fresh waters, or the metamorphosis
was more rapidly got rid of in the emigrants than in their fellows remaining in
the sea.
Animals without a metamorphosis would naturally transfer themselves more easily
to a new residence, as they had only themselves and not at the same time
multifarious young forms to adapt to the new conditions. But in the case of
animals with a metamorphosis, the mortality among the larvæ, always
considerable, must have become still greater under new than under accustomed
conditions, every step towards the simplification of the process of development
must therefore have given them a still greater preponderance over their
fellows, and the effacing of the metamorphosis must have gone on more rapidly.
What has taken place in each individual case, whether the species has
immigrated after it had lost the metamorphosis, or lost the metamorphosis after
its immigration, will not always be easy to decide. When there are marine
allies without, or with only a slight metamorphosis, like the Lobster as the
cousin of the Cray-fish, we may take up the former supposition; when allies
with a metamorphosis still live upon the land or in fresh water, as in the case
of Gecarcinus, we may adopt the latter.
That besides this gradual extinction of the primitive history, a
falsification of the record preserved in the developmental history takes
place by means of the struggle for existence which the free-living young states
have to undergo, requires no further exposition. For it is perfectly evident
that the struggle for existence and natural selection combined with this, must
act in the same way, in change and development, upon larvæ which have to
provide for themselves, as upon adult animals. The changes of the larvæ,
independent of the progress of the adult animal, will become the more
considerable, the longer the duration of the life of the larva in comparison to
that of the adult animal, the greater the difference in their mode of life, and
the more sharply marked the division of labour between the different stages of
development. These processes have to a certain extent an action opposed to the
gradual extinction of the primitive history; they increase the differences
between the individual stages of development, and it will be easily seen how
even a straightforward course of development may be again converted by them
into a development with metamorphosis. By this means many, and it seems to me
valid reasons may be brought up in favour of the opinion that the most ancient
Insects approached more nearly to the existing Orthoptera, and perhaps to the
wingless Blattidæ, than to any other order, and that the “complete
metamorphosis” of the Beetles, Lepidoptera, etc., is of later origin.
There were, I believe, perfect Insects before larvæ and pupæ; but, on the
contrary, Nauplii and Zoëæ far earlier than perfect Prawns. In
contradistinction to the inherited metamorphosis of the Prawns, we may
call that of the Coleoptera, Lepidoptera, etc. an acquired
metamorphosis.[2]
Which of the different modes of development at present occurring in a class of
animals may claim to be that approaching most nearly to the original one, is
easy to judge from the above statements.
The primitive history of a species will be preserved in its developmental
history the more perfectly, the longer the series of young states through which
it passes by uniform steps; and the more truly, the less the mode of life of
the young departs from that of the adults, and the less the peculiarities of
the individual young states can be conceived as transferred back from later
ones in previous periods of life, or as independently acquired.
Let us apply this to the Crustacea.
CHAPTER XII.
PROGRESS OF EVOLUTION IN CRUSTACEA.
According to all the characters established in the last paragraph, the Prawn
that we traced from the Nauplius through states analogous to Zoëa and
Mysis to the form of a Macrurous Crustacean appears at present to be the
animal, which in the section of the higher Crustacea (Malacostraca) furnishes
the truest and most complete indications of its primitive history. That it is
the most complete is at once evident. That it is the truest must be assumed, in
the first place, because the mode of life of the various ages is less different
than in the majority of the other Podophthalma; for from the Nauplius to the
young Prawn they were found swimming freely in the sea, whilst Crabs,
Porcellanæ, the Tatuira, Squilla, and many Macrura, when adult
usually reside under stones, in the clefts of rocks, holes in the earth,
subterranean galleries, sand, etc., not to mention other deviations in habits
such as are presented by the Hermit Crabs, Pinnotheres, etc.,—and
secondly and especially because the peculiarities which distinguish the Zoëa of
this species particularly from other Zoëæ (the employment of the anterior limbs
for swimming, the furcate tail, the simple heart, the deficiency of the paired
eyes and abdomen at first, etc.) are neither to be deduced from a
retro-transfer of late-acquired advantages to this early period of life, nor to
be regarded at all as advantages over other Zoëæ which the larva might have
acquired in the struggle for existence.
A similar development must have been once passed through by the primitive
ancestor of all Malacostraca, probably differing from that of our Prawn,
especially in the circumstance that it would go on more uniformly without the
sudden change of form and mode of locomotion produced in the latter by the
simultaneous sprouting forth and entering into action in the Nauplius of four
and in the Zoëa of five pairs of limbs. It is to be supposed that, not only
originally but even still, in the larvæ of the first Malacostraca, the new
body-segments and pairs of limbs are formed singly,—first of all the
segments of the fore-body, then those of the abdomen, and finally those of the
middle-body,—and, moreover, that in each region of the body the anterior
segments were formed earlier than the posterior ones, and therefore last of all
the hindermost segment of the middle-body. Of this original mode more or less
distinct traces still remain, even in species in which, in other respects, the
course of development of their ancestors is already nearly effaced. Thus the
abdominal feet of the Prawn-larva represented in Fig. 33, are formed singly
from before backwards, and after these the last feet of the middle-body; thus,
in Palinurus, the last two pairs of feet of the middle-body are formed
later than the rest; thus in the young larvæ of the Stomapoda the last three
abdominal segments are destitute of limbs, which are still wanting on the last
of them in older larvæ; and thus, in the Isopoda, the historically newest pair
of feet is produced later than all the rest. In the Copepoda this formation of
new segments and limbs, gradually advancing from before backwards, is more
perfectly preserved than in any of the higher Crustacea.[1]
The original development of the Malacostraca starting from the Nauplius, or the
lowest free-living grade with which we are acquainted in the class of
Crustacea, is now-a-days nearly effaced in the majority of them. That this
extinction has actually taken place in the way already deduced as a direct
consequence from Darwin’s theory, will be the more easily demonstrated,
the more this process is still included in the course of life, and the less
completely it is already worn out. We may hope to obtain the most striking
examples in the still unknown developmental history of the various Schizopoda,
Peneïdæ, and, indeed, of the Macrura in general. At present the
multifarious Zoëa-forms appear to be particularly instructive. Almost all the
peculiarities by which they depart from the primitive form of the Zoëa of
Penëus (Figs. 29, 30, 32), may in fact be conceived as transferred back
from a later period into this early period of life. This is the case with the
large compound eyes,—with the structure of the heart,—with the
raptorial feet in Squilla,—and with the powerful, muscular,
straightly-extended abdomen in Palæmon, Alpheus, Hippolyte, and the
Hermit Crabs. (In the latter, indeed, the abdomen of the adult animal is a
shapeless sac filled with the liver and generative organs, but it is still
tolerably powerful in the Glaucothoë-stage, and was certainly still more
powerful when this stage was still the permanent form of the animal.) It is
also the case with the abdomen of the Zoëæ of the Crabs, the Porcellanæ,
and the Tatuira, which is still powerful, although usually bent under the
breast; the two last swim tolerably by means of the abdomen, even when adult,
as do the true Crabs in the young state known as Megalops. It is the
case, lastly, with the conversion of the two anterior pairs of limbs into
antennæ. The second pair of antennæ, which, in the various Zoëæ always remains
a step behind that of the adult animal, is particularly remarkable. In the
Crabs the “scale” is entirely wanting; their Zoëæ have it indicated
in the form of a moveable appendage, which is often exceedingly minute. In the
Hermit Crabs a similar, usually moveable, spiniform process occurs as the
remains of the scale; their Zoëæ have a well-developed but inarticulate scale.
A precisely similar scale is possessed by the adult Prawns, in the Zoëæ of
which it exists still in a jointed form, like the outer branch of the second
pair of feet of the Nauplius or Penëus-Zoëa.
The long, spiniform processes on the carapace of the Zoëæ of the Crabs and
Porcellanæ are not to be explained in this way, but their advantage to
the larvæ is evident. Thus, for example, if the body of the Zoëa of
Porcellana stellicola (Fig. 24), without the processes of the carapace
and without the abdomen, which however is not rigidly extensible, is scarcely
half a line in length, whilst with the processes it is four lines long, a mouth
of eight times the width is necessary in order to swallow the little animal
when thus armed.[2] Consequently these processes of the carapace
may be regarded as acquired by the Zoëa itself in the struggle for existence.
The formation of new limbs beneath the skin of the larvæ is also to be referred
to an earlier occurrence of processes which originally took place at a later
period. The original course must have been that they sprouted forth in a free
form upon the ventral surface of the larva in the next stage after the change
of skin; whilst now they are developed before the change of skin, and thus only
come into action a stage earlier. In larvæ which, for other reasons, must be
regarded as more nearly approaching the primitive form, the original mode
usually prevails in this particular also. Thus the caudal feet (the
“lateral caudal lamellæ”) are formed freely on the ventral surface
in Euphausia and the Prawns with Nauplius-brood, and within the caudal
lamellæ in the Prawns with Zoëa-brood, in Pagurus and Porcellana.
A compression of several stages into one, and thereby an abridgement and
simplification of the course of development, is expressed in the simultaneous
appearance of several new pairs of limbs.
How earlier young states may gradually be completely lost, is shown by
Mysis and the Isopoda. In Mysis there is still a trace of the
Nauplius-stage; being transferred back to a period when it had not to provide
for itself, the Nauplius has become degraded into a mere skin; in Ligia
(Figs. 36, 37) this larva-skin has lost the last traces of limbs, and in
Philoscia (Fig. 38) it is scarcely demonstrable.
Like the spinous processes of the Zoëæ, the chelæ on the penultimate pair of
feet of the young Brachyscelus are to be regarded as acquired by the
larva itself. The adult animals swim admirably and are not confined to their
host; as soon as the specimens of Chrysaora Blossevillei, Less., or
Rhizostoma cruciatum, Less., on which they are seated, become the sport
of the waves in the neighbourhood of the shore, they escape from them, and are
only to be obtained from lively Acalephs. The young are helpless creatures and
bad swimmers; a special apparatus for adhesion must be of great service to
them.
To review the developmental history of the different Malacostraca in detail
would furnish no results at all correspondent to the time occupied by
it,—if our knowledge was more complete it would be more profitable. I
therefore abandon it, but will not omit to mention that in it many difficulties
which cannot at present be satisfactorily solved would present themselves. To
these isolated difficulties I ascribe the less importance, however, because
even a little while ago, before the discovery of the Prawn-Nauplius, this
entire domain of the development of the Malacostraca was almost inaccessible to
Darwin’s theory.
Nor will I dwell upon the contradictions which appear to result from the
application of the Darwinian theory to this department. I leave it to our
opponents to find them out. Most of them may easily be proved to be only
apparent. There are two of these objections, however, which lie so much on the
surface that they can hardly escape being brought forward, and these, I think,
I must get rid of.
“The peculiarities in which the Zoëæ of the Crabs, the Porcellanæ,
the Tatuira, the Hermit Crabs, and the Prawns with Zoëa-brood agree, and by
which they are in common distinguished from the larvæ of Penëus produced
from Nauplii, forces us (it might be said) to the supposition that the common
ancestor of these various Decapods quitted the egg in a similar Zoëa-form. But
then neither Penëus with its Nauplius-brood, nor even apparently the
Palinuri could be referred back to this ancestor. The mode of
development of Penëus and Palinurus, as also several peculiar
larvæ of unknown origin, but which are in all probability to be attributed to
Macrurous Crustacea, necessitate on the contrary the opposite supposition,
namely, that the different groups of the Macrura have passed from their
original to their present mode of development independently of each other and
also independently of the Crabs.” To this we may answer that the
occurrence of the Zoëa-form in all the above-mentioned Decapoda, its existence
in Penëus during the whole of that period of life which is richest in
progress and in which the wide gap between the Nauplius and the Decapod is
filled up, its recurrence even in the development of the Stomapoda, the
occurrence of a larval form closely approaching the youngest Zoëa of
Penëus in the Schizopod genus Euphausia,) and the reminiscence of
the structure of Zoëa, which even the adult Tanais has preserved in its
mode of respiration,—all indicate Zoëa as one of those steps in
development which persisted as a permanent form throughout a long period of
repose, perhaps through a whole series of geological formations, and thus has
also made a deeper impression upon the development of its descendants, and
formed a firmer nucleus in the midst of other and more readily effaced young
states. It cannot, therefore, surprise us that in transitions from the original
mode of metamorphosis to direct development, even when produced independently,
the larval life commences in the same way with this Zoëa-form in different
families, in which the earlier stages of development are effaced. But except
what is common to all Zoëæ, and what may easily be explained as being
transferred back from a later into this stage, the Zoëæ of the Crabs, for
example, agree with those of Pagurus and Palæmon in no single
peculiarity of structure which leads us to suppose a common inheritance.
Consequently we may apparently assume, without hesitation, that when the
Brachyura and Macrura separated, the primitive ancestors of each of these
groups passed through a more complete metamorphosis, and that the transition to
the present mode of development belongs to a later period. With regard to the
Brachyura, it may be added that in them this transition occurred only a little
later and indeed before the existing families separated. The arrangement of the
processes of the carapace, and, still more, the similar number of the caudal
setæ in the most different Zoëæ of Crabs (Figs. 19–23) prove this. Such
an accordance in the number of organs apparently so unimportant is only
explicable by common inheritance. We may predict with certainty that amongst
the Brachyura no species will occur which, like Penëus, still produces
Nauplius-brood.[3]
As we have already seen, Mysis and the Isopoda depart from all other
Crustacea very remarkably by the fact that their embryos are curved upwards,
instead of, as elsewhere, downwards. Does not so isolated a phenomenon as this,
it might be asked, in the sense of Darwin’s theory, indicate a common
inheritance? Does it not necessitate that we should unite as the descendants of
the same primitive ancestors, Mysis with the Isopoda on the one hand,
and on the other the rest of the Podophthalma with the Amphipoda? I think not.
Such a necessity exists only for those who estimate a peculiarity at a higher
value because it makes its appearance at an earlier period of the egg-life.
Whoever regards species as not created independently and unchangeably, but as
having gradually become what they are, will say to himself that, when the
ancestors of our Mysides came (probably much later than those of the
Amphipoda and Isopoda) to develop numerous body-segments and limbs whilst still
embryos, as they could no longer find room in the egg when extended straight
out, and were therefore compelled to bend themselves, this could only take
place either upwards or downwards, and whatever conditions may have decided the
direction actually adopted, any near relationship to either of the two orders
of Edriophthalma could hardly have taken part in it.
It may, however, be remarked, that the different curvature of the embryo in the
Amphipoda and Isopoda is so far instructive, as it proves that their present
mode of development was adopted only after the separation of these orders, and
that, in the primitive stock of the Edriophthalma, the embryos were, if not
Nauplii, at least short enough in the body to find room in the egg in an
extended position, like the larvæ of Achtheres enclosed by the
Nauplius-skin. On the other hand the uniformity of development that prevails in
each of the two orders—which is expressed in the Amphipoda for example in
the formation of the “micropylar apparatus,” in the Isopoda in the
want of the last pair of ambulatory feet—testifies that the present mode
of development has come down from a very early period and extends back beyond
the separation of the present families. In these two orders also, as well as in
the Crabs, we can hardly hope to find traces of earlier young states, unless it
be in the family of the Tanaidæ.[4] If any one will furnish me with an Amphipod
or an Isopod with Nauplius-brood, the existence of which would not be more
remarkable in independently produced species than that of a Prawn with
Nauplius-brood, I will abandon the whole Darwinian theory.
With regard to the Crabs, and also to the Isopoda and Amphipoda, we were led to
the assumption that, about the period when these groups started from the common
stem, a simplification of their process of development took place. This also
seems to be intelligible from Darwin’s theory. When any circumstances
favourable to a group of animals caused its wider diffusion and divergence into
forms adapting themselves to new and various conditions of existence, this
greater variability, which betrays itself in the production of new forms, will
also favour the simplification of the development which is almost always
advantageous, and moreover, exactly at this period, during adaptation to new
circumstances, as has already been indicated with regard to fresh-water
animals, this simplification will be doubly beneficial, and therefore, in
connexion with this, a doubly strict selection will take place.
So much for the development of the higher Crustacea.
A closer examination of the developmental history of the lower Crustacea is
unnecessary after what has been said in general upon the historical
significance of the young states, and the application of this which has just
been made to the Malacostraca. We may see, without further discussion, how the
representation given by Claus of the development of the Copepoda may pass
almost word for word as the primitive history of those animals; we may find in
the Nauplius-skin of the larvæ of Achtheres and in the egg-like larva of
Cryptophialus, precisely similar traces of a transition towards direct
development, as were presented by the Nauplius-envelope of the embryos
of Mysis and the maggot-like larva of Ligia, etc.
It will be sufficient to indicate an essential difference in the process of
development in the higher and lower Crustacea. In the latter all new
body-segments and limbs which insert themselves between the two terminal
regions of the Nauplius, are formed in uninterrupted sequence from before
backwards; in the former there is further a new formation in the middle of the
body (the middle-body), which pushes itself in between the fore-body and the
abdomen in the same way, as these have done on their part between the head and
tail of the Nauplius. Thus, that which appears probable even from the
comparison of the limbs of the adult animal, finds fresh support in the
developmental history, namely, that the lower Crustacea, like the Insects, are
entirely destitute of the region of the body corresponding to the middle-body
of the Malacostraca. It seems probable that the swimming feet of the Copepoda,
as also of the pupæ of Cirripedia and Rhizocephala, represent the abdominal
feet of the Malacostraca, that is to say, are derived by inheritance from the
same source with them.
It would be easy to weave together the separate threads furnished by the young
forms of the various Crustacea, into a general picture of the primitive history
of this class. Such a picture, drawn with a little skill, and finished in
lively colours, would certainly be more attractive than the dry discussions
which I have tacked on to the developmental history of these animals. But the
mode of weaving in the loose threads would still in many cases be arbitrary,
and to be effected with equal justice in various ways; and many gaps would
still have to be filled up by means of more or less bold assumptions. Those who
have not wandered much in this region of research would then readily believe
that they were standing upon firm ground, where mere fancy had thrown an airy
bridge; those acquainted with the subject, on the other hand, would soon find
out these weak points in the structure, but would then be easily led to regard
even what was founded upon well considered facts, as merely floating in the
air. To obviate these misconceptions of its true contents from either side, it
would be necessary to accompany such a picture throughout with lengthy, dry
explanations. This has deterred me from further filling in the outline which I
had already sketched.
I will only give, as an example, the probable history of the production of a
single group of Crustacea, and indeed of the most abnormal of all, the
RHIZOCEPHALA, which in the sexually mature state differ so
enormously even from their nearest allies, the Cirripedia, and from their
peculiar mode of nourishment stand quite alone in the entire animal kingdom.
I must preface this with a few words upon the homology of the roots of the
Rhizocephala, i.e. the tubules which penetrate from its point of
adhesion into the body of the host, ramify amongst the viscera of the latter,
and terminate in cæcal branchlets. In the pupæ of the Rhizocephala (Fig. 58)
the foremost limbs (“prehensile antennæ”) bear, on each of the two
terminal joints, a tongue-like, thin-skinned appendage, in which we may
generally observe a few small strongly refractive granules, like those seen in
the roots of the adult animal. I have therefore supposed these appendages to be
the rudiments of the future roots. A perfectly similar appendage, “a most
delicate tube or ribbon,” was found by Darwin in free-swimming pupæ of
Lepas australis on the last joints of the “prehensile
antennæ.” From the perfect accordance in their entire structure shown by
the pupæ of the Rhizocephala and Cirripedia, there can be no doubt that the
appendages of Sacculina and Lepas, which are so like each other
and spring from the same spot, are homologous structures.
Now in three species of Lepas, in Dichelaspis Warwickii and in
Scalpellum Peronii, Darwin saw, on tearing recently-affixed animals from
their point or support, that a long narrow band issued from the same point of
the antennæ; its end was torn away, and in Dichelaspis, judging from its
ragged appearance, it had attached itself firmly to the support. From this it
follows that this appendage in Lepas australis can hardly be anything
but a young cement-duct. If, therefore, the supposition that the appendages on
the antennæ of the pupæ of Rhizocephala are young roots be correct, the roots
of the Rhizocephala are homologous with the cement-ducts of the Cirripedia. And
this, strange as it may appear at the first glance, seems to me scarcely
doubtful. It is true that the act of adhesion of the Rhizocephala has never yet
been observed, but it is more than probable that they attach themselves, just
like the Cirripedia, by means of the antennæ, and that therefore the points of
attachment in the two groups indicate homologous parts of the body. From the
point of attachment in the Rhizocephala the roots penetrate into the body of
the host, whilst in the Cirripedia, the cement-ducts issue from the same point.
The roots are blind tubes, ramified in different ways in different species. The
cement-ducts in the basis of the Balanidæ likewise constitute a generally
remarkably complicated system of ramified tubes, with regard to the mode of
termination of which nothing certain has yet been made out. Individual cæcal
branches are not unfrequently seen even in the vicinity of the carina; and, at
least in some species, in which the cement-ducts divide into extremely numerous
and fine branchlets, forming a network which gradually becomes denser towards
the circumference of the basis, these seem nowhere to possess an orifice.
Now as to the question: How were Cirripedia converted by natural selection into
Rhizocephala?
A considerable number of existing Cirripedia settle exclusively or chiefly upon
living animals;—on Sponges, Corals, Mollusks, Cetaceans, Turtles,
Sea-Snakes, Sharks, Crustaceans, Sea Urchins, and even on Acalephs.
Dichelaspis Darwinii was found by Filippi in the branchial cavity of
Palinurus vulgaris, and I have met with another species of the same
genus in the branchial cavity of Lupea diacantha.
The same thing may have taken place in primitive times. The supposition that
certain Cirripedes might once upon a time have selected the soft ventral
surface of a Crab, Porcellana or Pagurus, for its dwelling-place,
has certainly nothing improbable about it. If then the cement-ducts of such a
Cirripede instead of merely spreading on the surface, pierced or pushed before
them the soft ventral skin and penetrated into the interior of the host, this
must have been beneficial to the animal, because it would be thereby more
securely attached and protected from being thrown off during the moulting of
its host. Variations in this direction were preserved as advantageous.
But as soon as the cement-ducts penetrated into the body-cavity of the host and
were bathed by its fluids, an endosmotic interchange must necessarily have been
set up between the materials dissolved in these fluids and in the contents of
the cement-ducts, and this interchange could not be without influence upon the
nourishment of the parasite. The new source of nourishment opened up in this
manner was, as constantly flowing, more certain than that offered by the
nourishment accidentally whirled into the mouth of the sedentary animal. The
individuals favoured in the development of the cement-ducts now converted into
nutriferous roots, had more than others the prospect of abundant food, of
vigorous growth, and of producing a numerous progeny. With the further
development, assisted by natural selection, of the roots embracing the
intestine of the host and spreading amongst its hepatic tubes, the introduction
of nourishment through the mouth and all the parts implicated in it, such as
the whirling cirri, the buccal organs, and the intestine, gradually lost their
importance, became aborted by disuse, and finally disappeared without leaving a
trace of their existence. Protected by the abdomen of the Crab, or by the shell
inhabited by the Pagurus, the parasite also no longer required the
calcareous test, in which, no doubt, the first Cirripedes settling upon these
Decapods rejoiced. This protective covering, having become superfluous, also
disappeared, and there remained at last only a soft sack filled with eggs,
without limbs, without mouth or alimentary canal, and nourished, like a plant,
by means of roots, which it pushed into the body of its host. The Cirripede had
become a Rhizocephalon.
If it be desired to form a notion of what our parasite may have looked like
when half way in its progress from the one form to the other, we may consult
the figures given by Darwin, (Lepadidæ Pl. iv, figs. 1–7) of Anelasma
squalicola. This Lepadide, which lives upon Sharks in the North Sea, seems,
in fact, to be in the best way to lose its cirri and buccal organs in the same
manner. The widely-cleft, shell-less test is supported upon a thick peduncle,
which is immersed in the skin of the Shark. The surface of the peduncle is
beset with much-ramified, hollow filaments, which “penetrate the
Shark’s flesh like roots” (Darwin). Darwin looked in vain for
cement-glands and cement. It seems to me hardly doubtful, that the ramified
hollow filaments are themselves nothing but the cement-ducts converted into
nutritive roots, and that it is just in consequence of the development of this
new source of nourishment, that the cirri and buccal organs are in the highest
degree aborted. All the parts of the mouth are extremely minute; the palpi and
exterior maxillæ have almost disappeared; the cirri are thick, inarticulate,
and destitute of bristles; and the muscles both of the mouth and cirri are
without transverse striation. Darwin found the stomach perfectly empty in the
animal examined by him.
Having reached the Nauplius, the extreme outpost of the class, retiring
furthest into the gray mist of primitive time, we naturally look round us to
see whether ways may not be descried thence towards other bordering regions. By
the structure of the abdomen in Nauplius we might be reminded, like Oscar
Schmidt, of the moveable caudal fork of the Rotatoria, which many regard as
near allies of the Crustacea, or at any rate of the Arthropoda; in the six feet
surrounding the mouth we might imagine an originally radiate structure, and so
forth. But I can see nothing certain. Even towards the nearer provinces of the
Myriopoda and Arachnida I can find no bridge. For the Insecta alone, the
development of the Malacostraca may perhaps present a point of union. Like many
Zoëæ, the Insecta possess three pairs of limbs serving for the reception of
nourishment, and three pairs serving for locomotion; like the Zoëæ they have an
abdomen without appendages; as in all Zoëæ the mandibles in Insects are
destitute of palpi. Certainly but little in common, compared with the much
which distinguishes these two animal-forms. Nevertheless the supposition that
the Insecta had for their common ancestor a Zoëa which raised itself into a
life on land, may be recommended for further examination.
Much in what has been adduced above may be erroneous, many an interpretation
may have failed, and many a fact may not have been placed in its proper light.
But in one thing, I hope, I have succeeded,—in convincing
unprejudiced readers, that Darwin’s theory furnishes the key of
intelligibility for the developmental history of the Crustacea, as for so many
other facts inexplicable without it. The deficiencies of this attempt,
therefore, must not be laid to the charge of the plan drawn out by the sure
hand of the master, but solely to the clumsiness of the workman, who did not
know how to find the proper place for every portion of his material.
INDEX.
(Numbers represent chapter number. Click on number and use 'Find' in the 'Edit'
menu of your browser. Numbers in parentheses denote number of references in the
chapter.)
Acanthonotus Owenii, 2.
Acanthosoma, 7.
Achæus, 7 (2).
Achtheres, 12.
—— percarum, 9 (2).
Allorchestes, 4, 8.
Alpheus, 6, 7, 12.
Amphilochus, 2, 8.
Amphipoda, 3, 6, 8 (2), 12.
Amphithoë, 2, 8.
Anceus, 8.
Anelasma squalicola, 12.
Anilocra, 6.
Aratus, 2.
—— Pisonii, 5.
Artemia, 9.
Asellus, 8.
Atylus, 8.
—— carinatus, 2.
Batea, 8.
Bodotria, 4, 8.
Bopyridæ, 8 (2).
Bopyrus, 8 (2).
Brachyscelus, 6, 8 (2), 9, 10 (2), 12.
—— crusculum, 8.
Brachyura, 12.
Branchiopoda, 9.
Calanidæ, 10.
Caligus, 8.
Caprella, 8 (3).
—— attenuata, 6.
—— linearis, 6.
Carcinus mænas, 7.
Caridina, 7.
Cassidina, 6, 8 (3).
Cerapus, 2, 8 (3).
Chalimus, 8.
Chondracanthus, 9.
Chthamalus, 9.
Cirripedia, 9 (2), 10.
Cladocera, 9.
Copepoda, 4, 9 (2), 10, 12.
Corophium, 8 (2).
—— dentatum, 8.
Corycæidæ, 10.
Crangon, 7.
Crayfish, 10.
Cryptoniscus planarioïdes, 8 (2).
Cryptophialus, 12.
—— minutus, 9.
Cuma, 8.
Cumacea, 8.
Cyclograpsus, 4, 5, 7.
Cyclopidæ, 10.
Cyclops, 9 (2), 10.
Cyclopsine, 9.
Cymothoa, 8.
Cymothoadiens, 8.
Cypridina, 10.
Cypris, 8, 10.
Cyrtophium, 2, 8, 10.
Cythere, 10.
Daphnia pulex, 8.
Dercothoë, 8.
Diastylidæ, 4, 8.
Dichelaspis Warwickii, 12.
Dulichia, 8 (2), 10.
Edriophthalma, 3, 6, 8.
Entomostraca, 9.
Entoniscus, 8.
—— cancrorum, 6, 8 (2).
—— porcellanæ, 6, 8 (2), 12.
Erichthus, 7.
Eriphia gonagra, 2, 5.
Euphausia, 7 (2), 10, 12 (2).
Eurynome, 7.
Evadne, 8.
Filograna, 11.
Gammarus, 8.
—— ambulans, 8.
—— Dugesii, 4.
—— puteanus, 6.
Gecarcinus, 7 (2).
Gelasimus, 2, 4 (2), 5, 7.
—— vocans, 5.
Glaucothoë Peronii, 7.
Grapsus, 5 (2).
Hermit Crabs, 7 (3), 12.
Hippa emerita, 7 (2).
Hippolyte, 7 (2), 12.
Hyperia galba, 8.
—— Latreillei, 8.
—— Martinezii, 8.
“Hypérines anormales et ordinaires,” 6, 8.
Idothea, 8 (2).
Insecta, 11.
Isopoda, 3, 6 (2), 8, 12.
Kepone, 8.
Læmodipoda, 6.
Lepas, 9 (2).
—— anatifera, 6.
—— australis, 9, 12.
Lernæodiscus porcellanæ, 9.
Lernanthropus, 9.
Lestrigonus, 8 (2).
Leucifer, 7, 8, 10, 12.
Leucothoë, 2, 8.
Ligia, 8 (2), 10, 12.
Lobster, 7, 10.
Lupea diacantha, 5.
Macrura, 7, 12.
Maia, 7 (2).
Megalops, 12.
Melita, 8.
—— anisochir, 2.
—— exilii, 2 (2), 4.
—— Fresnelii, 2 (3).
—— insatiabilis, 4 (2).
—— Messalina, 4 (2).
—— palmata, 2, 4.
—— setipes, 2.
—— valida, 2.
Microdeutopus, 3, 8.
Montagua, 8.
Mysis, 7, 8, 10 (3), 12 (2).
“Nauplius-larvæ”, 3 (3), 7, 8, 9 (3), 12 (2).
Nebalia, 9.
Niphargus, 6.
Ocypoda, 2, 5 (2), 7.
—— rhombea, 5.
Orchestia, 8 (2), 10.
—— Darwinii, 4 (2).
—— gryphus, 4.
—— sylvicola, 4 (2).
—— tahitensis, 4.
—— telluris, 4 (2).
—— Tucurauna, 8.
—— Tucuratinga, 8.
Orchestoidea, 8.
Pagurus, 12 (2).
Palæmon, 7 (3), 10, 12 (2).
Palinurus, 7, 10, 12 (2).
Peltogaster, 9.
—— socialis, 9.
Penëus, 3, 10, 12 (2).
—— setiferus, 7.
Persephone, 12.
Philoscia, 8 (2), 12.
Phronima, 6, 10.
—— sedentaria, 8.
Phryxus, 8.
Phyllopoda, 9.
Phyllosoma, 7 (2).
Pinnotheres, 7.
Podophthalma, 7.
Polyphemus, 10.
Pontellidæ, 10.
Porcellana, 7 (3), 10, 12 (2).
—— stellicola, 7, 12.
Porcellionides, 8.
Praniza, 8.
Prawns, 7, 12.
Protella, 8.
Protula, 11.
Pycnogonidæ, 9.
Ranina, 2, 5.
Rhizocephala, 9 (2), 10, 12.
Sacculina purpurea, 9 (5).
Scalpellum Peronii, 12.
Sergestes, 7.
Serpulæ, 11.
Sesarma, 2, 4, 5, 7.
Shrimps, 7.
Sphæroma, 8.
Squilla, 6, 7 (2), 12.
Talitrus, 8.
Tanais, 6 (2), 8 (2), 10, 12.
—— dubius, 4.
—— Dulongii, 3, 4.
Tatuira, 7 (3), 12.
Tetraclita porosa, 9 (2).
Trilobites, 9.
Xantho, 7.
Xiphosura, 9.
Zoëæ, 3 (2), 6, 7 (2), 12 (3).
End of the Project Gutenberg EBook of Facts and Arguments for Darwin, by Fritz Müller
*** END OF THIS PROJECT GUTENBERG EBOOK FACTS AND ARGUMENTS FOR DARWIN ***
***** This file should be named 6475-h.htm or 6475-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/6/4/7/6475/
Produced by Sue Asscher
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org
Section 3. Information about the Project Gutenberg Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.


























